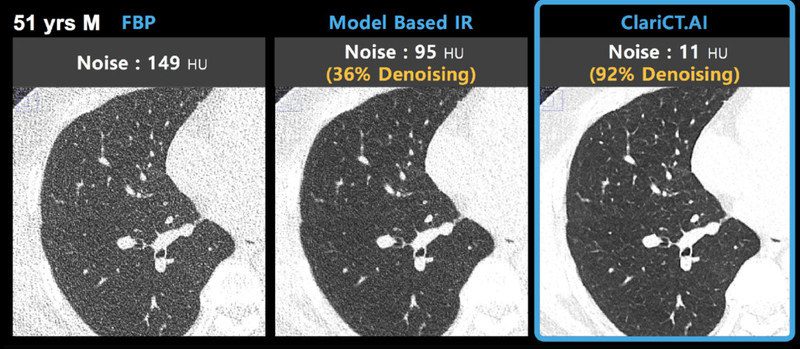
ClariPi, a leading AI medical imaging company, is presenting its AI-powered low-dose CT denoising solution, ClariCT.AI along with new innovative AI imaging solutions at RSNA 2021. ClariCT.AI is a vendor-agnostic software solution offering a superb clarity of CT images scanned with substantially reduced radiation dose, both FDA-cleared and CE-marked.
Trained with world’s largest image dataset over 1 million, its deep-learned Clarity Engine clears image noise safely while enhancing underlying structures to a level of regular dose images from the noisy ultra-low-dose CTs. Hyun-Sook Park, co-CEO of ClariPi said, “We are proud to see more clinical studies being published proving the efficacy of ClariCT.AI. We hope to collaborate with more partners to open the door to the age of ultra-low-dose CT where patients get CT scans without radiation anxiety any longer.”
ClariPi presented its new innovations at the AI Theater under the topic of ‘Bringing the Power of AI into Ultra-Low-Dose CT Imaging: ClariPi Inc.’ on Sunday, November 28. Harry Park, president of Global Sales & Marketing at ClariPi USA said, “We debuted another innovative product ClariACE, a software-based iodine contrast boost solution to address the need to minimize contrast medium side-effects in contrast-enhanced CT scans.” “On top of these, we also introduced ClariPulmo, a fully automated AI-powered 3D reporting solution for COVID-19 pneumonia and emphysema, along with ClariOsteo, an AI-powered precision bone healthcare solution both with low-dose and ultra-low-dose CT images.”
“With the Nuance AI Marketplace, we are one step closer to our vision- implementation is simplified, having the ClariCT.AI on the Nuance AI Marketplace will insure easy accessibility through cloud-based distribution and deployment.” said Jong-Hyo Kim, CTO and co-CEO of ClariPi.