Cook Medical has received a contract with Vizient for endoscopy devices. This new agreement will allow Cook to continue to offer our endoscopy devices at negotiated pricing terms to healthcare facilities that are members of Vizient. With the agreement from Vizient, which is the largest member-driven healthcare performance improvement company in the country, Cook looks forward to making endoscopy products more accessible for physicians and patients across the United States.
For almost 60 years, Cook Medical has collaborated with physicians to develop innovative devices to improve lives around the world. We brought our pioneering model for minimally invasive devices to the field of gastrointestinal (GI) endoscopy to diagnose and treat diseases throughout the gastrointestinal tract. The FDA has granted multiple Cook Endoscopy products FDA Breakthrough Device Designations, including our technologies for hemostasis and endoscopic ultrasound. Now, with this contract, endoscopy products will be more accessible to more patients.
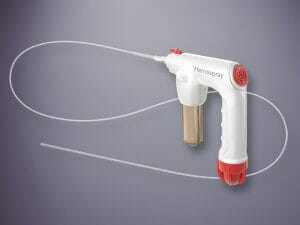
This new Vizient agreement becomes effective April 1, 2023. The agreement will include Cook’s full line of endoscopy medical devices, including Hemospray® Endoscopic Hemostat. The contract will also include products from the following product categories:
- Biliary access (long wire & short wire)
- Biliary / pancreatic therapy
- Biopsy, cytology & polypectomy
- Extraction, dilation, drainage stenting
- Stricture management
- Tissue management
- Esophageal, gastric, colonic
- Enteroscopy
- Hemostasis
- Endoscopic ultrasound
- Endobronchial ultrasound
- Endohepatology
- Endosurgery
“We are honored to have been chosen as an Endoscopy contracted supplier with Vizient. With over 40 years of expertise in the gastroenterology specialty, Cook Medical looks forward to continuing to work with Vizient members and the physicians that treat patients with our endoscopy medical devices,” said Rick Simms, Cook Medical National GPO Account Executive.
Contracting with Cook Medical has several benefits for hospital systems and healthcare facilities. Cook is dedicated to responsible product creation and consumption as we care for the planet. To learn more about our earth-conscious values, you can read about our initiatives here.
To learn more about our breakthrough products and other initiatives, visit Cook’s Endoscopy page.