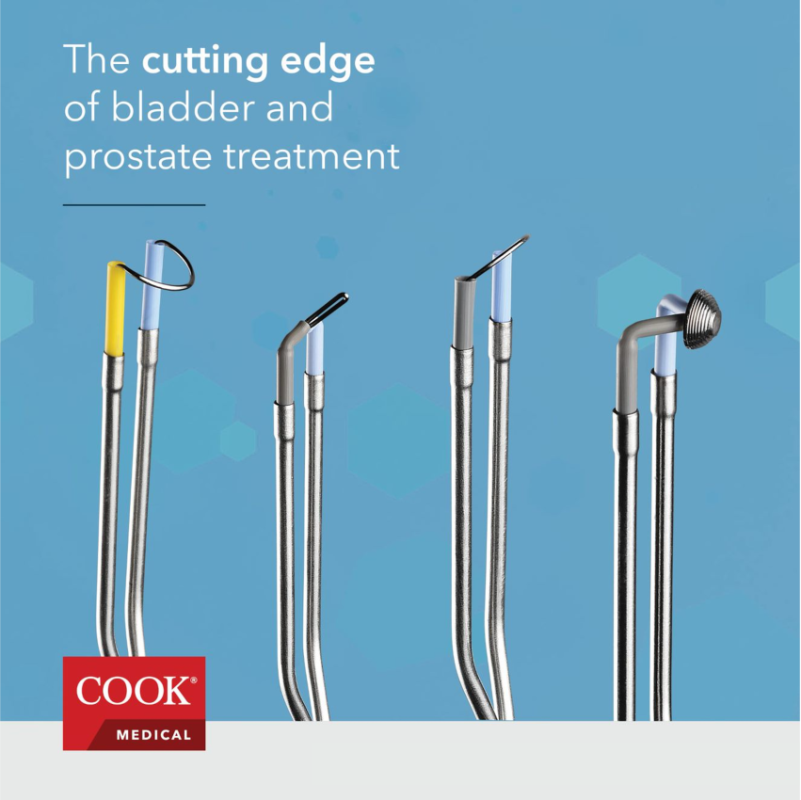
Cook Medical has launched a new, streamlined portfolio of urological bipolar electrodes in the U.S. This portfolio includes the products that urologists use most frequently to focus on daily electrode needs when performing procedures on the bladder and prostate.
The bipolar electrodes portfolio includes a total of seven products. The portfolio is streamlined to include the most frequently used and in-demand products in this category. Six of the products are configurations indicated for use in transurethral resection, ablation and soft tissue removal of the prostate and bladder and where hemostasis is required:
- A bipolar transurethral bladder loop, which is angled at 136° to accommodate the anatomy of the bladder
- Four transurethral cutting loops—12° and 30° options, each available in medium and large sizes
- A bipolar transurethral needle electrode
The seventh product is the Bipolar Transurethral Plasma Disc®, which is made for electro-vaporization in urological procedures to help with vaporization of the prostate and coagulation. The patented concentric, multi-tiered disc design by Omnitech Systems, Inc., provides concentrated current density to be more energy efficient while still supporting current flow and plasma ignition.2
The distal wires of each electrode tip (except for the transurethral needle electrode) are made from a platinum-iridium alloy, which extends from the leading edge past the stabilizer. The platinum-iridium alloy is reinforced by being crimped deep into the body of the electrode for durability. The electrodes have a shelf life of five years, providing additional stock and supply benefits.
“We listened to physicians about obstacles they were facing when trying to perform resection and ablation procedures, such as benign prostatic hyperplasia (BPH). For example, we learned that most bladder loops on the market were difficult to use around the bladder, so we offer a bladder loop angled at 136° to improve efficiency of surgery. With the seven products in the streamlined bipolar electrodes portfolio, we aim to help physicians focus on treating patients with products they use frequently,” said Rob Faulkner, senior director of Cook Medical’s Urology specialty.
For a product brochure and to learn more about Cook’s bipolar electrodes products, visit the product information page.
Plasma Disc is a registered trademark of Omnitech Systems, Inc.