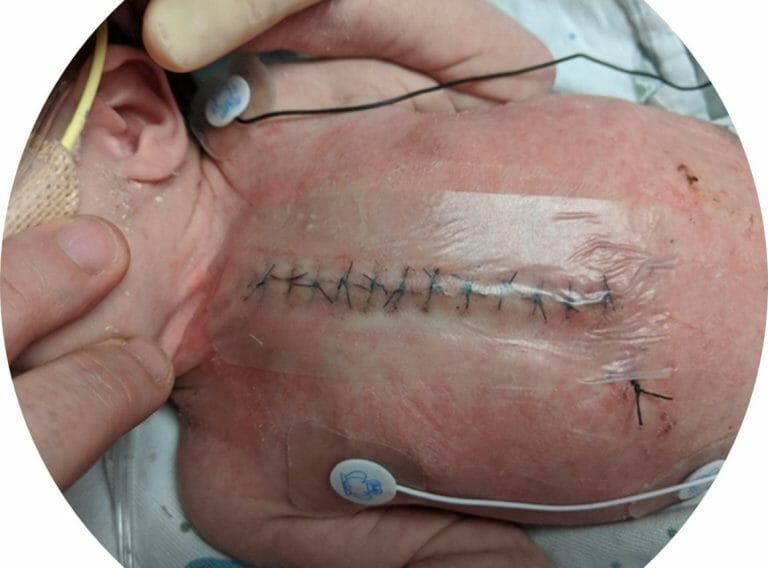
Covalon Technologies Ltd. (the “Company” or “Covalon”) (TSXV: COV) (OTCQX: CVALF), an advanced medical technologies company, today announced that its SurgiClear® dressing played a key part in an infection prevention bundle that significantly reduced surgical wound infections in a two-year quality improvement study recently published by Intermountain Primary Children’s Hospital, a not-for-profit children’s hospital associated with the University of Utah and part of the Intermountain Healthcare system.1 Intermountain Primary Children’s Hospital is recognized as one of the top children’s hospitals in the United States.
Describing the impact of the bundled intervention, published in The Annals of Thoracic Surgery, the authors from Intermountain Healthcare report that the annual rate of total surgical wound infections decreased from 2.83% in 2019 to 1.15% in 2021. The bundled approach used to improve perioperative infection prevention practices included implementation of SurgiClear as a main practice change.
Jeremy Harman, PA-C, an author of the study, commented, “The trifecta of breathable barrier protection, antimicrobials, and the ability to monitor the site is what makes this a great dressing. It’s easier for us to assess the wound and is more comfortable for patients.”
The aim of the project was to reduce the rate of surgical wound infections to less than 1.5% in pediatric cardiothoracic surgery patients. Surgical wound infections are a common complication of cardiac surgery, associated with increased length of hospital stay, readmissions, higher cost of care and increased mortality. Pediatric cardiology centers across the U.S. focusing on process improvement are investing in preventative strategies and quality improvement protocols to decrease the risk of surgical site infections. Advanced technology, such as Covalon’s SurgiClear, can help to standardize post-operative wound care.
“It is great to see another institution sharing their experience with our SurgiClear dressing through publication,” said Brian Pedlar, CEO. “At Covalon we’re committed to providing better experiences for patients and clinicians, and this work completed by Intermountain Primary Children’s Hospital shows how the best practices and products come together to do just that, helping the most vulnerable heal.”
SurgiClear is a dual-antimicrobial clear silicone surgical dressing that provides full site visibility, allowing for inspection of surgical wound sites, while minimizing contact and repetitive dressing removal. Its dual-antimicrobial silicone technology allows for broad spectrum protection from both gram-positive and gram-negative bacteria that is gentle on even the most sensitive skin.
In addition to SurigClear, Covalon’s other infection prevention solutions include:
- VALGuard® – an FDA listed, transparent, environmental barrier designed to protect catheter hubs and line connections from external contaminants and gross contamination, including body fluids and other secretions. It incorporates a quick-release pull strip for fast access to infusion hubs and for easy removal.
- IV Clear® – an antimicrobial vascular access dressing that offers complete transparency at and around the insertion site for easy daily assessment. It uses a soft silicone adhesive to preserve the skin’s barrier function and minimize skin injuries and incorporates safe amounts of antimicrobials, without sacrificing efficacy, to protect against chemical irritation.
Reference
- Glenn, E. T., Harman, J. R., Marietta, J., Lake, J., Bailly, D. K., Ou, Z., Griffiths, E. R., & Ware, A. L. (2022). Impact of a Surgical Wound Infection Prevention Bundle in Pediatric Cardiothoracic Surgery. The Annals of Thoracic Surgery. https://doi.org/10.1016/j.athoracsur.2022.08.045