CSA Medical, Inc., today announced that all subjects have been enrolled in the RejuvenAir® System study Characterizing the Mechanism of Action of Metered Cryospray for the Treatment of Patients with Chronic Obstructive Pulmonary Disease with Chronic Bronchitis.
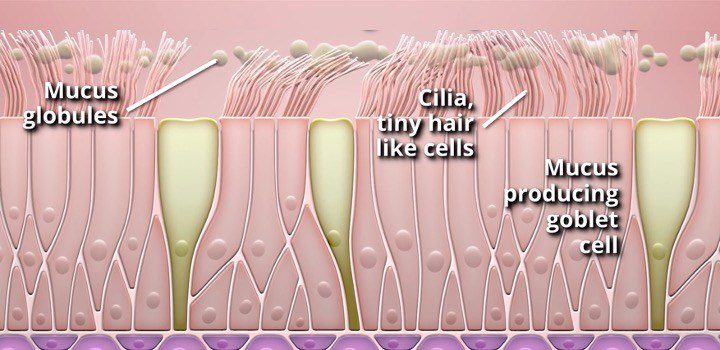
The aim of this study is to determine the mechanism by which Metered CryoSpray (MCS) reduces the density of goblet cells responsible for over-producing mucus and observe re-growth of healthy cilia in the airways.
“In the previous study, we saw meaningful improvements in patient symptoms and quality of life,” said Professor Pallav Shah M.D., from the Royal Brompton, London. “What makes this study different is that we will now be able to provide the cellular rationale for the remodeling response we observed in the feasibility study after treatment with RejuvenAir. In other words, we will prove re-epithelialization and regrowth of healthy airway tissue.”
CSA Medical, the developer of the RejuvenAir System, is currently conducting a pivotal study in twenty sites across the United States. The SPRAY-CB Study is actively enrolling subjects who suffer from the debilitating symptoms of Chronic Bronchitis.
COPD is a long-term, progressive, irreversible lung disease that, over time, makes it difficult to breathe. Chronic Bronchitis (CB), the largest subset of COPD, is characterized by a chronic productive cough. The SPRAY-CB study is investigating a minimally invasive device therapy, Metered CryoSpray (MCS), which utilizes the RejuvenAir® System, a revolutionary cryosurgical device which applies a precise thermal dose of extremely cold, -196◦C liquid nitrogen to targeted areas within the lungs through an outpatient bronchoscopic procedure.
“Together with the final data from the SPRAY-CB pivotal study, we endeavor to understand not only that metered cryospray reduces the symptoms of chronic bronchitis, but also answer how it works to rejuvenate airways for a better quality of life. Current medications only manage those symptoms, we anticipate the RejuvenAir® System will be the first minimally invasive treatment option for this population,” stated Heather V. Nigro, Senior Vice President of Regulatory, Quality and Clinical Affairs at CSA Medical.