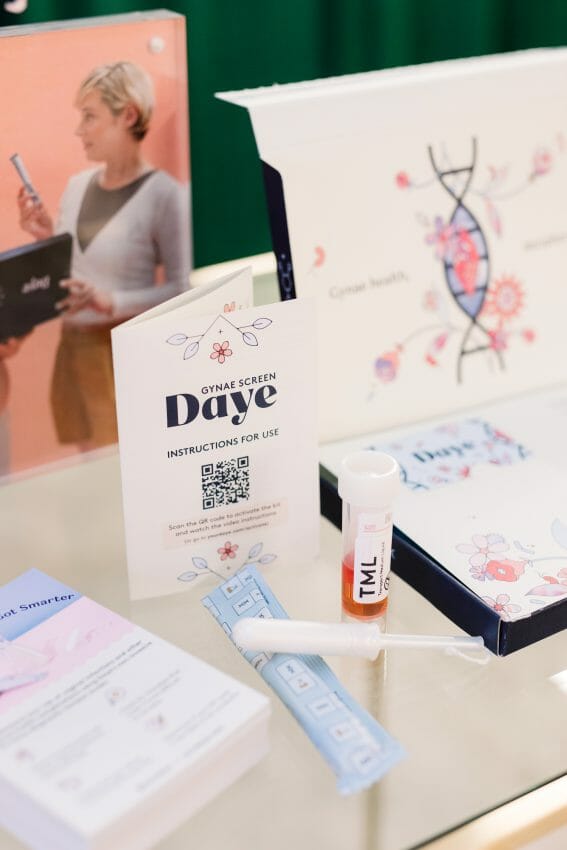
Today, female-founded gynaecological health startup Daye and clinical trial startup Lindus Health are announcing the launch of their clinical trial for a ‘Diagnostic Tampon’ which will enable women and assigned female at birth (AFAB) individuals to carry out HPV and STI tests from the comfort of their own homes.
Daye, who invented the world’s first Cannabidiol (CBD) Tampon, has now developed a Diagnostic Tampon for sample collection and analysis for HPV and STI detection.
It is estimated that approximately 8 in 10 people will be infected with HPV at some point in their lives. However, many women and (AFAB) individuals face both practical and emotional barriers to screening, including difficulties in arranging appointments, fear and embarrassment.
Supporting Daye’s mission to close the gender health gap, its Diagnostic Tampon has the potential to transform women’s experience when being screened for HPV and STIs, enabling comfortable, noninvasive at-home testing. Using a tampon can also improve the accuracy of the test, as a tampon collects a bigger and more comprehensive sample than a swab.
The trial will see 375 patients across the UK and Italy test menstrual tampons to help support Daye’s product commercialisation with payors by assessing the diagnostic tampon’s efficacy, safety, and acceptability.
Lindus Health is responsible for full trial delivery and supporting protocol drafting and submission packages. This includes recruiting and consenting participants, data collection through their proprietary trial management software, and project management.
Commenting on the trial, Michael Young, Co-Founder of Lindus Health said: “With 8 in 10 people estimated to contract HPV in their lifetimes, and with complications leading to a range of cancers, it is vital that the industry looks to break down the barriers to testing and early detection.
“Through this trial, we hope to be instrumental in delivering a more comfortable alternative to screening for women – which could, in turn, save many lives”.
Valentina Milanova, Founder of Daye, said: “Time is of the essence when it comes to HPV and STI detection, as catching them early can help to prevent some of the negative effects and complications — but unfortunately many people still face practical and emotional barriers to getting a diagnosis.
“We are committed to making it easier for women and AFAB individuals to take control of their health, and at-home testing has a huge role to play in speeding up access to diagnosis and treatment.
“Nothing could be more familiar to women than the humble tampon. After completing clinical trials with over 600 participants, we are looking forward to starting clinical investigations with Lindus to further assess the role they could play in HPV and STI testing at a global scale.”