Reflow Medical, Inc., a medical device company focused on cardiovascular disease, announces that the first patient was successfully treated and enrolled in the DEEPER REVEAL investigational device exemption (IDE) clinical trial (NCT05358353) at Advanced Cardiac & Vascular Centers (ACV) in Grand Rapids, Michigan. The Bare Temporary Spur Stent System was granted Breakthrough Designation status by the U.S. Food & Drug Administration (FDA) in August 2021.
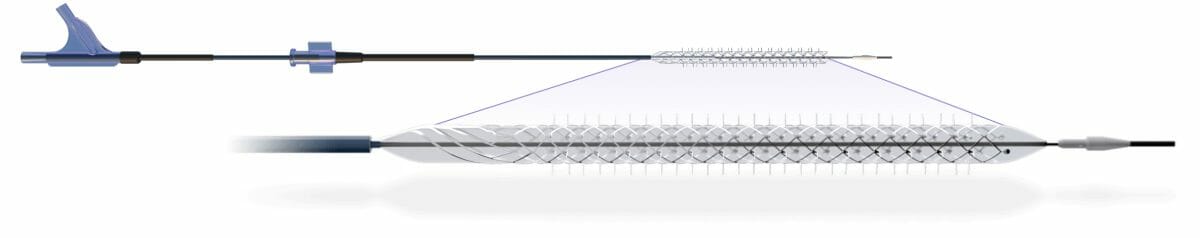
The DEEPER REVEAL trial is a prospective, single-arm, multicenter study that will examine the efficacy and safety of the Spur Stent for the treatment of vascular lesions in patients with critical limb ischemia (CLI). The device is a temporary self-expanding stent scaffold with radial spikes used to treat the diseased vessel wall without leaving anything behind. It is expected that 130 patients will be enrolled in the study at up to 50 centers.
Jihad A. Mustapha, MD, FACC, FSCAI, one of the co-Principal Investigators for the study and ACV’s President & Chief Executive Officer, Director of Endovascular Interventions, thanked the team at ACV Center for their efforts in the recruiting and enrollment process.
“The Spur Stent was created to fulfill an unmet need in treating patients with CLI. The ease with which this device can be directed to the lesion, deployed and retrieved should be acknowledged. We are excited to be studying this breakthrough technology,” he said.
The study is also being led by co-Principal Investigator Jay Mathews, MD, MS, FACC, FSCAI, of Bradenton Cardiology Center in Bradenton, Florida, and Mahmood K. Razavi, MD, MS, FSIR, FSVM, of Vascular and Interventional Specialists of Orange County, Inc. in Orange, California. According to Dr. Mathews,
“The Spur Stent has shown encouraging results to date in trials in Europe, notably in achieving acute luminal gain and reducing vessel recoil. We’re looking forward to enrolling our first patients in the DEEPER REVEAL study here in Florida.”
Co-Principal Investigator Dr. Razavi, MD, MS, FSIR, FSVM, joined in congratulating Reflow Medical on enrolling the first patient.
“This is a pioneering technology that has already shown promising results in previous trials treating below-the-knee disease,” he said. “The Spur Stent technology provides a novel approach for controlled treatment of calcified vessel walls. The fact that it leaves no metal behind will preserve future treatment options.”
Isa Rizk, CEO and Co-Founder of Reflow Medical, commented on enrolling the first patient in the DEEPER REVEAL study. “This is a major step on the path to US approval for the Spur Stent. We’re certainly grateful to partner with our principal investigators and looking forward to working with all the future hospitals and institutions as we gain traction and enrollments for continued clinical validation.”
The DEEPER REVEAL trial continues to build on Reflow Medical’s commitment to improving outcomes for patients suffering from critical limb ischemia, for which there are limited solutions to date.