Author: Jaume Miras
Chief Strategy Officer
GPAINNOVA Barcelona, Spain
Due to medical needs, there is a whole industry focused on the creation of implants for different parts of the body.
 Osteosynthesis implants are used to restore broken bones and to offer relief to an existing medical condition. From dental to vertebrae, shoulder joints or pelvis; there have been many implantology improvements. The importance of having the right implant goes beyond the creation of a metal piece that imitates a part of the body. Today’s engineers are constantly researching to make these implants even better, more functional and to increase their lifespan. For instance, in order to improve long-lasting results, recent studies have shown that improving the surface’s quality on an implant will increase implant functionalities as well as their lifespan.
Osteosynthesis implants are used to restore broken bones and to offer relief to an existing medical condition. From dental to vertebrae, shoulder joints or pelvis; there have been many implantology improvements. The importance of having the right implant goes beyond the creation of a metal piece that imitates a part of the body. Today’s engineers are constantly researching to make these implants even better, more functional and to increase their lifespan. For instance, in order to improve long-lasting results, recent studies have shown that improving the surface’s quality on an implant will increase implant functionalities as well as their lifespan.
Implant Limitations Nowadays
Even though technology has made it possible to achieve significant progress in the design and manufacture of implants for osteosynthesis, there is still room for improvement as the implant can fail and even damage the patient due to several factors. Implants tend to suffer from fretting corrosion due to the nature of the movement inside the body, the fluids inside the body and the mechanical stress of its functionality. Implant performance is also affected by the polishing and finishing processes as they impact the fatigue and tensile strengths and on some occasions, the implant is not entirely biocompatible.
A severe incidence in manufacturing different implants refers to the large variety of post-processing steps, even involving manual labor. When a patient is using an implant, it must be manufactured in a way that prevents the patient from additional risks. Some of the parts just need to be slightly rounded and clean. Some of them need intensive processing so they have to be polished to work correctly. If an implant is manufactured negligently or without considering surface quality, it can cause corrosion, premature fracture, and consequent rejection. Corrosion, in this case, is causing metal to enter the patient’s bloodstreams and resulting in blood poisoning.
The typical process to finish implants at large scale is based on chemical-mechanical processes like robotized belt polishing or abrasive polishing. These types of process work generating friction to the piece and against a generic abrasive. This usually involves the use of water and solvents to clean the piece. Although there are some other procedures that involve dry polishing, this other type of polishing primarily refers to circular vibrators. They are commonly used to round the edges and polish the surface of the implant. Those processes tend to present some issues as they are error-prone. It’s hard to certify a specific success-scale as they don’t offer consistency on results. Current processes are not enough reliable and require additional manual rework to achieve the desired finishing, manufacturing also implies the scrapping of faulty parts. Moreover, this equipment requires additional costly peripheral equipment to treat the water and sludge contaminated with metals that require specific maintenance and negatively affecting our environment.
But, What Makes DLyte Different?

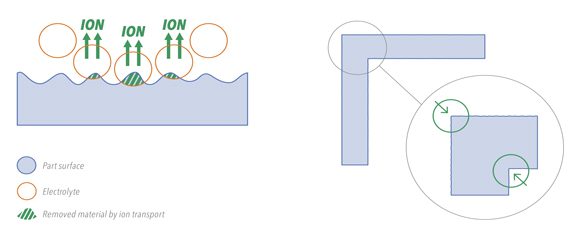
With this new system, DLyte precisely offers what the implant industry was looking for. This system focused on the post-process of the implant allows taking a step forward in the finishing of medical device parts. Automated, accurate, friendly to use and clean, this process is reinventing the way manufacturing of implants is done.
Competitive Advantages
DLyte system based on dry electropolishing address the main needs of the implant industry such as shape and tolerances preservation, biocompatibility, corrosion resistance enhance, automation and reduction of complexity of current manual and multistep processes, high output with a very compact equipment, no dust and waste emissions, no need of peripheral water recycling equipment, clean and non-hazardous process and applicable for fragile parts.
Biocompatibility Proved

Based on results published, the materials processed (CoCr and Ti) with DLyte System meets the acceptance criteria of study, i.e.:
- The viability has been superior than 70% in all concentrations tested compared to the blank control.
- The extract at 50% of the test sample has shown greater or equal viability than the extract at 100%.
- The mean value of blanks has not differed by more than 15% of the average value of all blanks.
The materials processed can be considered non-cytotoxic. The study has been made according to the specifications of standard UNE-EN-ISO 10993-5:2009.
Resistance to Corrosion

Recent studies evaluated the corrosion resistance of a SS316 piece processed with DLyte compared with one processed with liquid electropolishing bath. The results show that DLyte achieves better corrosion resistance than liquid electropolishing. The dry electropolished sample analyzed has lower surface roughness and more luster then liquid electropolished sample.
- The dry Electropolishing sample has better protection to corrosion until 6 hours of immersion in corrosion potential test.
- The dry Electropolishing sample shows a corrosion rate between 4 to 15 times slower than the liquid Electropolishing sample in polarization resistance test.
Cost-effective and process complexity reduction
Current multi-step implant finishing process is very costly and implies grinding, tumbling, use of a mechanical robot and manual buffing. This process also makes impossible to standardize the product due to manual’s labor inconsistencies. The mentioned arguments imply that the current state-of-art offers inconsistent results. The current process also has high lead times and higher costs and there is a need for waste management and water recycling with the consequent peripheral equipment requirement. And requires cleaning and corrosion enhance the process to be able to be implanted. With DLyte there is a drastic total process time and steps reduction compared to traditional finishing process. There is a significant improvement related to quality and labor cost per piece and there is no need for an additional corrosion resistance to enhance the process. These benefits have led DLyte to be approved by top implant manufacturers.
Fragile Parts Finishing

DLyte electropolishing can be used for finishing fragile parts with a wide range of applications from slight deburring to high gloss polishing as the media is non-abrasive and during the process, a high vibration is applied to reduce the friction between the media and the surface. Compared to the liquid electropolishing DLyte is easier to control as it is less aggressive treatment.
Additive Manufacturing

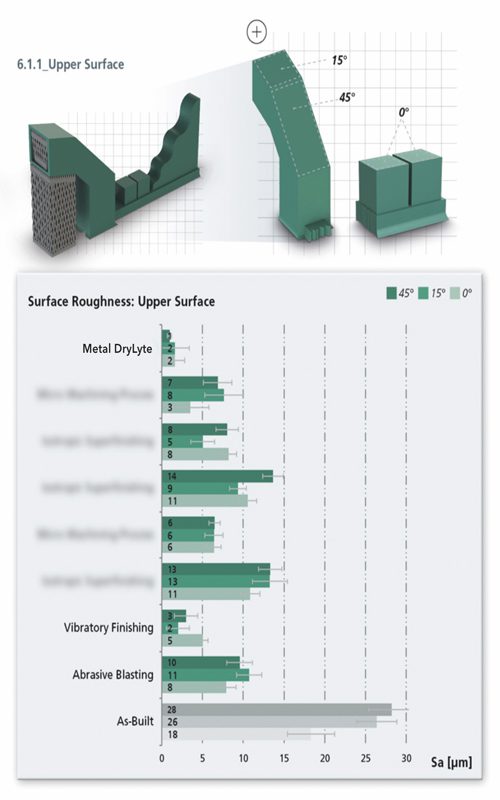
One study conducted by Fraunhofer IAPT focused on the additive manufacturing industry shows that DLyte performs at least two times better surface quality than the next-best alternative between the 7 most relevant post-processing technologies.
Additionally, DLyte was the winner of the post-processing award in 2018 by the TCT Awards[1] because of the excellence of the process to threaten additive manufacturing metal parts.
To Conclude
The main reason why DLyte is a disruptive solution for surface finishing in the medical device industry is the fact that this technology can better handle the real industry needs. DLyte system innovates to improve the manufacturing process, reducing error-prone, decreasing costs and shortening lead times. DLyte machines also allow to manufacture in an automated process. Therefore, it is also suitable for large production still delivering consistent results. The arrival of DLyte to the medical device industry covers the lack of innovation in post-processing and opens the door to a substantial improvement of quality and lifespan of medical implants responding to a demand given today’s longevity in our society.
Reference
[1] TCT awards recognizes ground breaking technology, collaborative projects, industry leaders and innovators.