World-renowned eyelid and facial cosmetic surgeon Dr Adam Scheiner has opened a new, solo practice.
Establishing a solo cosmetic laser surgery practice represents the next chapter in Dr. Scheiner’s groundbreaking career. After two decades of delivering patient rejuvenations as a valued partner of Tampa Eye Clinic’s physician team, Dr. Scheiner says, “It’s time to take the next step and focus on delivering my innovative RESET ® procedures in a setting that’s dedicated to patient transformations.”
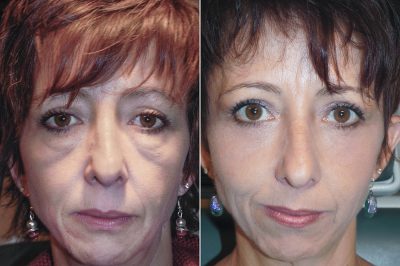
Dr Adam Scheiner is the only physician in the world to offer RESET, his proprietary advanced deep resurfacing procedures. These surgeries go beyond reducing signs of aging: they reset the skin and restore the patient’s eyes to their appearance from years earlier.
Dr. Scheiner’s new practice is in Tampa, Florida, where he has been treating patients from around the world for 25 years. RESET for Festoons is his signature surgery, offering the only real solution for a condition that others refuse to treat or mistreat, often making Festoons look worse. He also uses advanced lasers for full face rejuvenation, and recently perfected RESET for Upper Eyelids, a procedure that resurfaces eyelid skin after blepharoplasty, tightening and smoothing this delicate skin.
Dr Adam Scheiner said
“Practicing on my own with a complete focus on cosmetic patients has been a goal for a long time. My team and I are excited about concentrating on providing RESET ® procedures for eyelid and facial transformations. I’m also hoping to continue developing innovative, transformative procedures.”
All three RESET surgeries offer natural results that turn back the clock by decades, reducing the signs of aging and resetting the skin’s age.