Dr Greta Dalle Luche, PhD writes ….
London-born Parasym™, a biotechnology company specialised in clinical neuromodulation, reports positive results for the treatment of heart failure with preserved ejection fraction (HFpEF) from the University of Oklahoma’s randomized, placebo-controlled pilot study using Parasym’s non-invasive neuromodulation device. The results revealed improvements in both quality of life (QoL) and physiological parameters, paving the way for the first non-invasive and non-pharmacological treatment for this condition.
HFpEF has been recently defined as “the single largest unmet need in cardiovascular medicine based on prevalence, poor outcomes and the absence of treatment options until now”. Despite affecting 60 million people worldwide, there are very few treatment options available to patients. In the US, there are currently no approved devices and limited pharmaceuticals indicated for HFpEF. The promising results of the recently published clinical trial using transcutaneous vagus nerve stimulation to treat HFpEF change the paradigm of the current standard of care for heart failure and highlight the potential for neuromodulation to lead the way for a new electroceutical era.
STUDY HIGHLIGHTS
The sham‐controlled, double‐blind, randomized clinical study, published in the Journal of the American Heart Association, was led by Dr. Stavros Stavrakis, MD, Ph.D. of University of Oklahoma Health Sciences Center and evaluated the effectiveness of non-invasive transcutaneous vagus nerve stimulation (nVNS) to treat patients with HFpEF. Fifty-two participants received active (n=26) or sham (n=26) targeted transcutaneous vagus nerve stimulation via the ear for one hour daily for three months using Parasym’s patented neuromodulation technology. The protocol adherence was monitored and echocardiography, a 6-minute walk test, quality of life (QoL) assessed by the Minnesota Living with Heart Failure Questionnaire, and serum cytokines were assessed at baseline and as outcome parameters after 3 months. Significant improvements in QoL, global longitudinal strain, and inflammatory cytokines, tumor necrosis factor-α (TNF- α ) and IL-8, were shown in the active group compared to the sham at 3 months from the start of the treatment. Interestingly, a significant correlation was observed between the decrease between TNF- α levels and the global longitudinal strain improvement. These placebo-controlled results are extremely promising as they show a significant improvement in QoL and significant changes in tangible physiological parameters and cardiac mechanisms (supporting the hypothesized mechanism of action) for a patient population that is completely underserved by the currently available treatments, and doing so through a non-invasive, scalable, and low-risk intervention with little or no side effects. Parasym’s neuromodulatory technology paves the way for a new era of heart failure treatment.
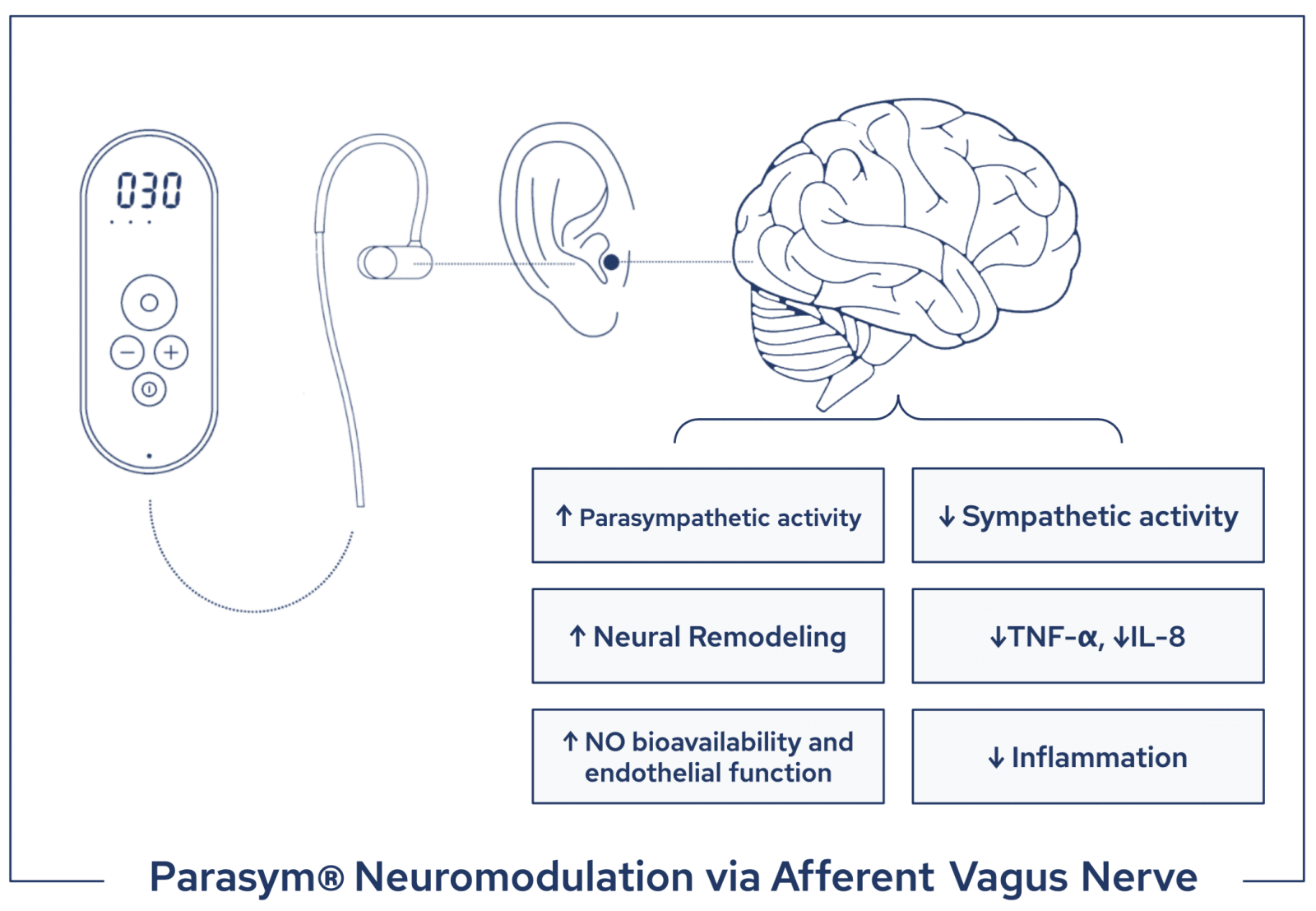
THE DEVICE
Parasym’s patented neuromodulation technology is engineered and designed to provide accessible, at-home targeted nVNS. The device consists of a battery-powered external pulse generator the size of a smartphone and an accompanying earpiece which is worn on the ear without piercing the skin. The device is portable and rechargeable and allows for quick application and removal after each session. Targeted low-level electrical impulses at specific optimized parameters are transmitted transcutaneously to the auricular branch of the vagus nerve to modulate key mechanisms that are implicated in heart failure. In this study, the device was used for one hour daily, although previous studies using the same device observed significant changes in physiological parameters after as little as 10 minutes of use.
HOW IT WORKS
Parasym’s treatment targets the auricular afferent branch of the vagus nerve with optimized electric impulses that rebalance the autonomic nervous system promoting parasympathetic activity. Increases in parasympathetic activity downregulate unhealthy immune response mechanisms through the “cholinergic anti-inflammatory pathway”. Targeted nVNS stimulates the release of the neurotransmitter acetylcholine which binds at the α7 subunit of the nicotinic acetylcholine receptor and attenuates the production of pro-inflammatory cytokines, such as TNF- α and IL-8. As systemic inflammation decreases, endothelial function is ameliorated by an increase in endothelial nitric oxide synthase activity. Restored bioavailability of nitric oxide allows for microvascular relaxation and improves cardiovascular function. A recent sham-controlled randomized clinical trial led by the same investigative team used Parasym nVNS to treat patients with paroxysmal atrial fibrillation (AF) obtaining a significant 85% decrease in AF burden in the active group compared to sham after 6 months from the start of the treatment. Overall, the currently available clinical data suggest that nVNS reduces systemic and myocardial inflammation and diastolic dysfunction at the basis of the pathogenesis of HFpEF and other cardiovascular diseases and is on the trajectory to provide a new revolutionary class of treatments utilizing electroceutical technology.
HFpEF
Heart failure is a chronic, highly debilitating disease associated with high rates of mortality and related comorbid conditions, such as hypertension, AF, coronary artery disease. Heart failure encompasses diverse disease phenotypes characterized by ineffective pumping of blood by the heart coupled with systemic consequences involving other major organs (e.g. lung and kidneys). HFpEF indicates that the left ventricle is not filling with blood effectively, while the ejection fraction is “preserved” which means it is generally 50% or more, as opposed to heart failure with reduced ejection fraction, HFrEF, where the ejection fraction is generally less than 50%. Patients diagnosed with HFpEF make up for approximately half of the total heart failure patients and suffer the same level of mortalities and comorbidities as those with HFrEF although they are left with fewer treatment options. There is a serious unmet need for low-risk, effective treatment options that can address HFpEF and in particular have a positive impact on QoL outcomes.
The current goal of treatment for HFpEF is the reduction of symptoms and risk of hospital admission. This often involves addressing related comorbidities rather than providing HFpEF-directed treatment. To date, there are no FDA-approved pharmacological options that have shown a statistically significant improvement in quality of life for the treatment of HFpEF and few pharmacological interventions have been shown to decrease mortality in HFpEF. Of the few treatment options available, which include pharmacological and surgical interventions, only a limited number are meaningfully improving quality of life and most have considerable side effects.
The abovementioned recent randomized controlled trial showed not only significant improvement in patient QoL but also a restoration of healthy cardiac mechanics and a decrease in markers of inflammation. Notably, these results show that a remote, home-based treatment such as Parasym’s neuromodulation can provide a much-needed treatment option for patients as well as relieve the huge burden heart disease puts on our healthcare systems. nVNS is generally recognized as safe, being granted international approvals for other classes of diseases. No device-related side effects for Parasym’s auricular nVNS were observed during the HFpEF clinical trial, and only very rare and limited side effects (such as mild discomfort at the application site) had been previously recorded for the device. Importantly, Parasym’s treatment can also be used alongside pharmaceutical intervention without causing drug interactions, allowing for combined therapies for improved patient outcomes. Parasym’s device could take the lead as the first and only non-invasive, low-risk and minimal side effect option for heart failure treatment.
About Parasym and the author
Dr. Greta Dalle Luche, PhD. Research and Development Lead at Parasym. Founded in 2015 by Sophie Dundovic and Nathan Dundovic, Parasym™ is a neurotechnology company pioneering a new frontier in medicine – electroceuticals. Parasym specializes in innovative neuromodulation products that improve health and performance, and has over 60 clinical partnerships across 4 continents, including Harvard, Chicago Medicine, and Oklahoma University. Focusing on the heart and brain, Parasym’s neuromodulation technology has demonstrated significant clinical results in the treatment of high-impact diseases such as Heart Failure, Atrial Fibrillation, and long COVID, with over 1,000,000 treatment sessions completed. Parasym also helps support the environment by donating 1% of revenue to fund grassroots environmental initiatives. For more information about Parasym’s latest products, visit Nurosym.
