February 1, 2021
EarlySense, the global leader in contact-free, continuous monitoring solutions across the healthcare continuum, announced today that it has sold proprietary contact-free continuous monitoring technology to Hillrom (NYSE: HRC).
Early Sense notes under terms of the agreement, EarlySense will receive licensing for all intellectual property and technology sold to Hillrom for use outside the hospital, as well as a cash consideration of $30 million, potential payments based on the achievement of certain commercial milestones, and a portion of Hillrom’s equity investment in EarlySense.
“We set out to save lives and improve care by implementing AI-based contact-free patient monitoring in healthcare facilities across the globe,” said Matt Johnson, CEO of EarlySense. “We made great progress by becoming the standard of care in Hillrom’s flagship Centrella® Smart+ hospital bed. Our technology has already monitored more than one million patients and helped clinicians save tens of thousands of lives, and we expect to accelerate this significant impact as a result of this technology sale to Hillrom.
“The COVID-19 pandemic has created historic opportunities for smart health technologies outside the hospital, and this transaction will allow us to continue serving our global customer base and apply our clinically proven technology to this high-growth sector. Our more than one hundred million patient-monitored hours uniquely position us to deliver intelligent, predictive, and patented solutions that elevate remote patient care without increasing the risk of harmful infection,” added Johnson.
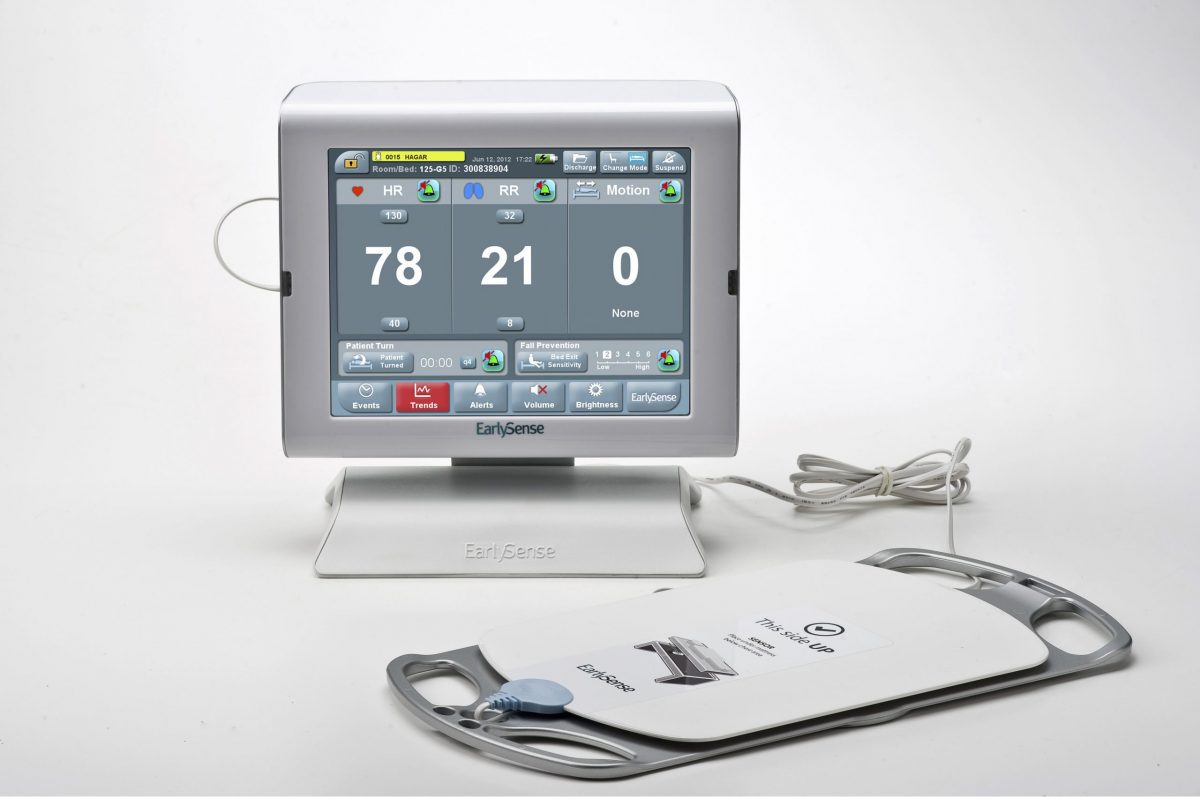
EarlySense technology allows for the continuous (more than 100 times per minute) monitoring of patient heart and respiratory rates without ever touching the patient. The technology also alerts clinicians to potential patient deterioration events much earlier than traditional monitoring methods. This technology serves as the engine for EarlySense remote patient monitoring devices, helping bring hospital-grade patient analytics to care settings outside the hospital.
“EarlySense’s contact-free continuous monitoring technology provides caregivers with a full picture of patient health, allowing for intervention at the earliest signs of patient deterioration,” said Hillrom President and CEO John Groetelaars. “We look forward to bringing this critical technology to additional customers around the world as we deliver on our vision of Advancing Connected Care™.”
Wells Fargo Securities served as exclusive financial advisor to EarlySense and Goldfarb & Seligman and Polsinelli served as legal counsel.