EchoNous, the leader in portable AI-guided ultrasound tools and software, announced it has raised $60 million in funding from Kennedy Lewis Investment Management (“Kennedy Lewis”), a leading opportunistic credit manager with a strong track record of supporting innovative life sciences companies.
The investment from Kennedy Lewis combines senior secured debt and equity and builds on the support of KKR, which has been EchoNous’ principal investor since 2015.
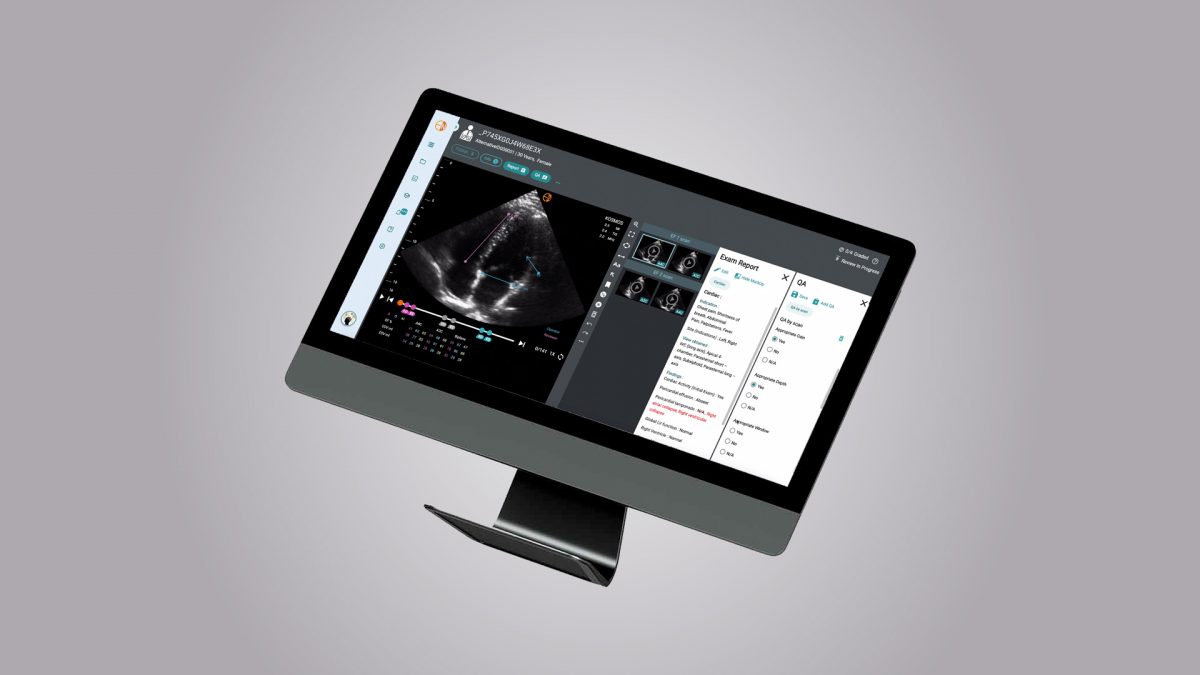
Proceeds from the financing will help fuel the commercial launch of Kosmos, EchoNous’ flagship device. Kosmos is the first AI-guided point-of-care ultrasound (POCUS) system to provide diagnostic-quality imaging on par with larger, less portable, and more expensive imaging machines. Kosmos’ system enables systolic heart function assessment, and it is the only handheld POCUS device to offer continuous-wave Doppler capability, a feature with important applications for cardiology. In addition, Kosmos is the only product of its size and in its category to meet HIPAA requirements for data collection, storage, and transmission.
Rich Gumer, Managing Director at Kennedy Lewis and head of the firm’s Life Sciences strategy, said: “We are thrilled to partner with EchoNous on its next phase of growth. EchoNous has an exceptional track record in developing high quality AI-guided ultrasound devices which are more intelligent, accessible, and easier to use. Kosmos’ superior imaging quality, mobility and affordability have the potential to significantly improve patient care and lower healthcare costs which are the core tenets of Kennedy Lewis’ investment ethos in life sciences.”
“This investment marks a turn toward an exciting future for POCUS in which clinical bedside medicine is driven by higher-performance ultrasound technologies that are easy to implement and cost effective,” added Kevin Goodwin, CEO of EchoNous. “To make this vision a reality, we value experienced partners who can see that future as clearly as we do. We could not be happier to have Kennedy Lewis’ support.”