January 20, 2021
Serenno Medical, developer of medical devices for patient monitoring in a hospital setting, announced today that Elcam Medical, a leading producer of medical devices, has invested $1.5 million in the Company and will also manufacture SentinelTM, Serenno’s urine output and intra-abdominal pressure digital monitoring device for the detection of acute kidney injury (AKI). Elcam Medical join existing investors Alon Medtech and serial medical device inventor, entrepreneur and investor, Dr. Shimon Eckhouse.
The partnership announced today combines Serenno’s unique solution with Elcam’s superior manufacturing experience to deliver high quality products at a competitive price, that will enable the use of Sentinel in a variety of hospital environments.
Continuous kidney function assessment allows the early detection of AKI, a common condition in hospitalized patients that significantly increases risk of mortality during and after hospitalization. Accurate measurement of urine output (UO) is clinically accepted as the best method for monitoring changes in kidney function. However, UO is currently monitored intermittently and manually by ICU staff, therefore acute changes in urine flow are difficult to detect. Thus, kidney injury is often detected relatively late, sometimes after it is impossible to prevent further progression.
Beyond the high death toll associated with kidney failure routinely in the ICU, the recent spread of COVID19 had greatly increased prevalence of, and death from AKI, while vastly increasing the demand and exposure risk on medical professionals and ICUs worldwide.
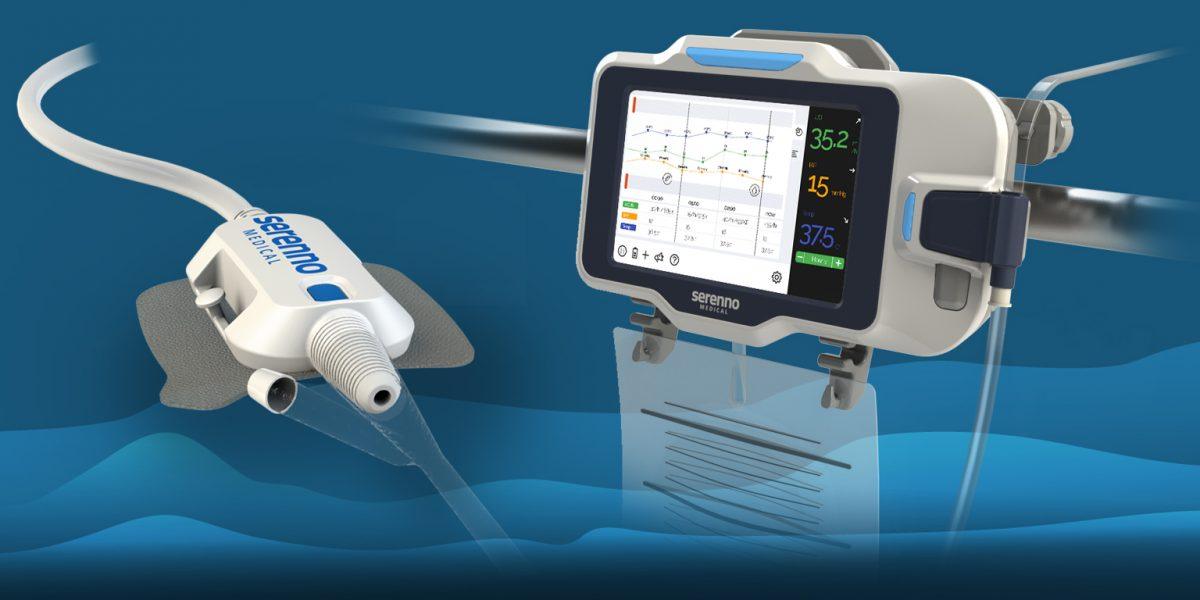
Sentinel offers a simple and cost-effective solution for the precise, continuous measurement of urine volume and flow rate in real time. The system promotes early detection of kidney injury, while there is still time to intervene and prevent further damage. It aims to automatically and accurately detect small changes in kidney function, allowing remote detection by the medical staff, thus reducing both patient and caregiver risk.
“The investment by Elcam Medical is a strong endorsement, and will allow us to swiftly move toward commercialization of our device,” said Tomer Lark, Serenno’s co-founder and CEO. “Elcam’s world class production capabilities and experience, will enable mass production and increased market access of Sentinel. Our plan is to begin deployment of the devices in several US hospitals towards the end of 2021, in the hope to eventually reduce kidney failure risk for every patient at ICUs.”
Dr. Shimon Eckhouse, Serenno’s Chairman, added, “We are pleased with Elcam’s investment, which marks the first commitment in Serenno’s larger planned current financial round. This partnership with Elcam is an excellent indication of the high degree of innovation and the significance of Serenno’s solution for the advancement of monitoring technologies. We are confident that Elcam’s leadership position in the global point-of-care market will play a critical role in bringing Serenno’s exciting technology to ICUs and patients around the world.”
“Our investment in Serenno and the establishment of a production line for its device is aligned with Elcam’s strategy to cooperate with promising Israeli startups to deliver products with added value for the patient,” said Igal Kohn, Elcam Medical’s CEO. “Elcam Medical has joined Serenno as a board member and we look forward to taking part in the Company’s continued successes.”