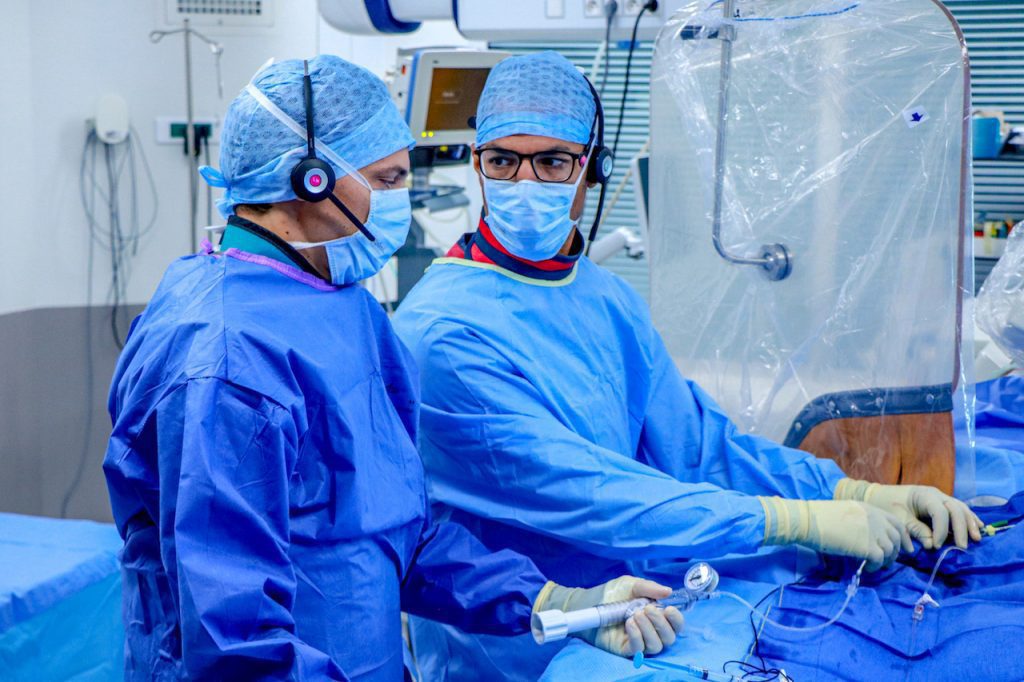
Electroducer, the designer and developer of the innovative medical device Electroducer Sleeve®, which treats heart valve disease, today announces that it has raised €3 million ($3.65M) from private investors and French public stakeholders.
The funds will be used for marketing its new technology, which has been proven to be safe and effective in a pilot study involving 60 patients in France.
Prior to their official release, the results of the clinical trial were presented on May 18, 2021, at EuroPCR, an international interventional cardiology conference – attended by nearly 10,000 doctors. The study was a world first; it represents a quantum leap forward within this field of medicine.
Major clinical requirement
The technique, which was invented in the early 2000s by Professor Alain Cribier, requires a temporary pacemaker, which is inserted directly into the patient’s heart. This is a key step, as it requires doctors to ‘pause’ the heart and ensure that the replacement valve is positioned correctly. However, in 2–5% of cases there are some risks and major complications involved for patients, which can include death.
One-of-a-kind technology, based on a proven medical technique
The Electroducer Sleeve is a device with no equivalent anywhere in the world. It is based on a medical technique called Direct Wire Pacing (DWP®), which was invented by the company’s founder, Dr. Benjamin Faurie, and used for the first time ever in 2011. The technique is now regularly employed by French doctors when replacing heart valves. It removes the traumatic and painful step where a pacemaker would be temporarily placed into the patient’s heart, replacing this with stimulation sent via the ‘guidewire’, an instrument used to move the valve into position. In simple terms, a low intensity electric current is generated along the guidewire, safely stimulating the heart during the procedure, with no risk of complications for the patient.
Proven clinical benefits
The ‘EASY TAVI’ study, published in the prestigious Journal of the American College of Cardiology (JACC) in 2019, proved that this technique works better than the conventional approach. Direct Wire Pacing significantly reduced per- and post-operational trauma for patients and reduces the length of the procedure. For the doctor, the technique means a simpler procedure and better working conditions, with less exposure to X-rays, which are known to cause serious injuries such as cancer. Lastly, using this device also saves hospitals money; a reduction of roughly 12% per procedure —as some devices are no longer needed and the reduced risk of complications means this can be an outpatient procedure. The technique also proved beneficial during the COVID-19 pandemic by limiting the amount of time patients were in hospital, increasing the number of patients being treated and freeing up medical and paramedical resources.
Ready-to-use device, offering safe and effective treatment
However, Direct Wire Pacing has some technical limitations that hinder widespread adoption. The purpose of the Electroducer Sleeve is to simplify, enhance and expand the use of this revolutionary technique using a turnkey method with proven clinical benefits.
The results of the pilot study, which involved 60 patients in three French centers, showed that the use of this device is both safe and effective. In particular, it increases patient tolerance, with no pain caused during stimulation. It also makes the technique used by the doctor more easily repeatable.
Dr. Benjamin Faurie, chairman, chief executive officer and founder of Electroducer, said: “I’m proud to see the technique that I established ten years ago is now widespread in France and is continuing to be developed through a new medical device that is 100% safe and effective for the patient. Initial reports are very promising and indicate a shallow learning curve, as the solution is universal and needs no other special equipment. We’re very much looking forward to receiving marketing authorization, which will allow the technique to be rolled out worldwide and save a lot of lives.”
Potential market of nearly 1.5 million procedures per year worldwide
The Electroducer Sleeve is expected to come onto the market in the US and Europe during 2022, following FDA authorization in the US and CE marking in Europe.
Initially, the Electroducer Sleeve device will be used for aortic valve replacement procedures and for more complex coronary procedures treating the narrowing of the arteries that supply the heart. Subsequently, the technology will also be applied to procedures involving the mitral and tricuspid heart valves.