Enspectra Health, is a digital health company dedicated to pioneering the field of virtual biopsies.
Enspectra Health announced today that it has received a Phase IIB award of $4.0 million under the National Institutes of Health‘s Small Business Innovation Research Program (SBIR) for the continued development of its novel imaging technology to aid in the evaluation of nonmelanoma skin cancers (NMSCs).
NMSCs account for more than 75 percent of all cancers diagnosed in the United States. NMSC can cause destruction to the skin and underlying tissue within months and if poorly diagnosed, can lead to unnecessary biopsies or death. Today’s gold standard for diagnosis is a biopsy which requires excision of skin followed by microscopic evaluation. Of the 16 million biopsies conducted annually, approximately half are not cancerous and could be avoided.
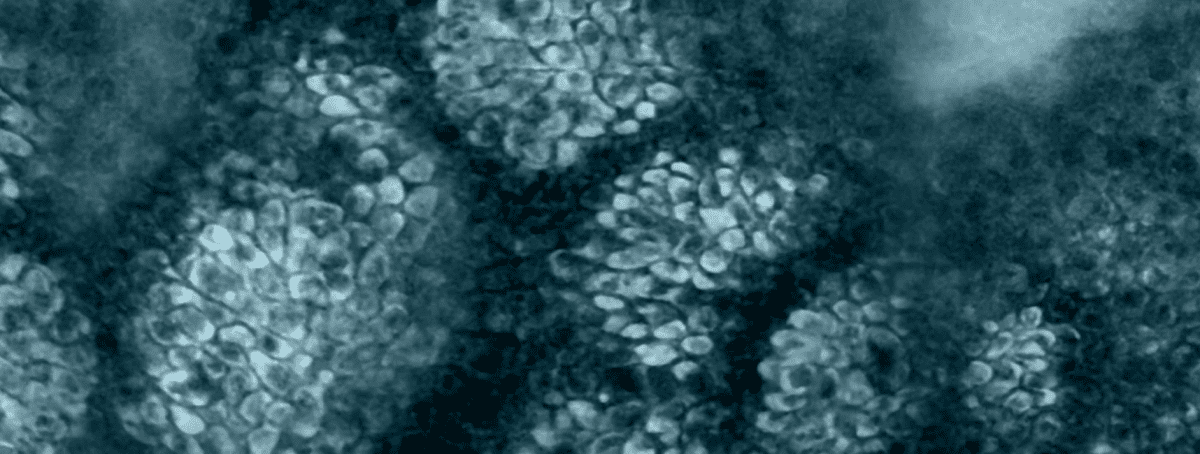
Enspectra Health is pioneering a new field of virtual biopsies, providing high-resolution images of cellular structures in living skin without the need for an incision. With real-time evaluation across the surface of the skin, Enspectra’s technology holds the potential to reduce unnecessary biopsies and allow clinicians to examine suspicious lesions earlier.
Funds from the NIH grant will enable Enspectra Health to accelerate product development and generate clinical evidence to commercialize its platform technology.