Epitel, Inc., a digital health company developing a wearable, wireless EEG monitoring platform for seizure detection, announced today the closing of a $12.5 million Series A financing for initial pilot commercialization and further development of its proprietary platform.
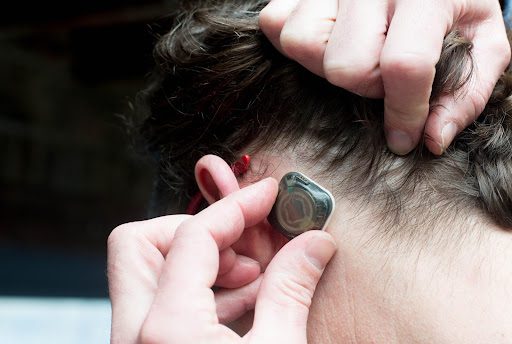
Epitel received FDA clearance for its first product, a wireless and wearable EEG (brain wave monitor) sensor, and remote access software known as REMI® for use within hospital emergency rooms and critical care units. REMI first received clearance from the U.S. Food & Drug Administration in 2021.
Two-thirds of the U.S. population lack ready access to EEGs and most emergency departments lack the capability to adequately monitor EEG (Ward et al., 2012. Neurocrit Care. 16(2):232-40.) REMI, Epitel’s first FDA-cleared product solves this problem with wearable, wireless sensors that can be rapidly and easily applied by a nurse or hospital technician.
EEG data is then immediately connected to a cloud-based software platform available to neurologists to review and monitor for seizures at any time from any location. Because the Epitel System is wearable and wireless, it can continue to monitor the patient continuously for 48 hours during their hospital journey.
“Epitel’s first FDA-cleared product, REMI, has the potential to revolutionize the diagnosis, treatment, and management of seizures within the hospital. With Epitel, patients, no matter their geography, may have access to essential EEGs during the most critical times of need,” said Mark Lehmkuhle, Ph.D., Chief Executive Officer of Epitel.
He added, “We intend to further expand our product pipeline for use outside the hospital by people living with epilepsy and other seizure conditions. We are honored to have the support of Catalyst Health Ventures, Genoa Ventures, and a strong investment syndicate in our first financing.”
Catalyst Health Ventures (CHV) and Genoa Ventures co-led the Series A financing along with participation from Dexcom and OSF Ventures. Wavemaker 360, MedMountain Ventures and Salt Lake City Angels also participated in the round. In conjunction with this close, Vikram Chaudhery, Ph.D., of Genoa Ventures, and Joshua Phillips of CHV have been appointed to the Board of Directors.
Andy Rasdal, founding CEO of Dexcom, and Kim Kamdar, Ph.D., of Domain Associates join the board as Executive Chairman and Independent Director respectively. Prior to Series A, the company has been primarily grant-backed with funding from the NIH and Epilepsy Foundation totaling over $7.5 million.
“It is time that EEGs for the brain become as accessible as EKGs for the heart to patients throughout the country. For too long essential neurological services have been inaccessible to large parts of our population,” said Dr. Chaudery, Principal with Genoa Ventures. “Genoa Ventures is excited to support Epitel in their journey to become the leader in remote seizure management and transform how clinicians monitor and diagnose seizures in the ED, ICU, ambulatory, and at-home settings. Most importantly we were excited about Epitel’s potential for broad impact across all these settings and changing the way we think about long-term brain health management.”
“We are excited to partner with the Epitel team to support bringing this disruptive technology to the market,” said Mr. Phillips, Managing Partner of CHV. “Epitel’s technology platform stands out as the first EEG system that may with further development more seamlessly support a seizure patient from the hospital to the home while integrating into existing physician and hospital workflows. With Epitel, the future is brighter for people living with acute and chronic neurological conditions.”