Fujitsu Limited today announced that it has started joint clinical research with the University of Tokyo Hospital to verify the effectiveness of artificial intelligence technology to estimate abnormal heart movements based on electrocardiogram(1) data. The research is planned to commence at the University of Tokyo Hospital from October 25.
Since December 2019, Fujitsu has been making progress with research and development of proprietary AI technology(2) in collaboration with the University of Tokyo Hospital. This research draws on data from patients that have visited the University of Tokyo Hospital to date and includes approximately 630,000 pieces of electrocardiographic data and data from approximately 140,000 cardiac ultrasounds (echocardiography)(3). This initiative has now succeeded in detecting patients with abnormal heart movements with high accuracy.
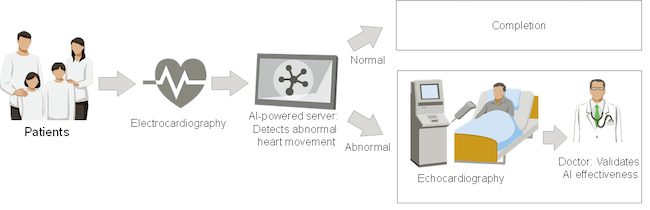
The AI will be used in clinical field research to estimate the presence or absence of abnormal heart movements based on the data of patients undergoing electrocardiographic procedures at the University of Tokyo Hospital. Patients identified by the AI as having abnormal heart movements will undergo echocardiography, and the effectiveness will be verified by comparing the results of doctors’ diagnosis with the results predicted by the AI. Fujitsu will leverage this AI to detect heart disease at an early stage to prevent progression to serious illness in patients and strengthen its vision of “Healthy Living”, promoting well-being for people throughout society.
This initiative will be introduced at Fujitsu ActivateNow 2021, a global event to be held from October 12 in online format.
Background
Heart disease is the second leading cause of death in Japan (4), and electrocardiography, which detects abnormalities in the heart muscle and irregular pulses based on the waveform of electrical pulses in the heart, is widely used as a diagnostic tool to detect heart disease at an early stage. It remains difficult, however, to detect abnormalities in the shape and movement of the heart using only electrocardiograms. In medical practice, in addition to electrocardiography, doctors use a stethoscope to detect abnormal heart sounds (murmurs, arrythmia, etc.) based on the patient’s description of their symptoms, followed by echocardiography to detect abnormalities in heart shape and movement. However, because echocardiographic diagnostics can only be performed in a limited number of facilities with specialized doctors and laboratory technicians, it is difficult to offer this for all patients. This makes early detection difficult, and when problems are detected, the progression of the disease may already be advanced. In the treatment of heart disease, early detection and appropriate treatment are a vital issue in medical practice.
Since December 2019, in collaboration with a research group headed by Dr. Katsuhito Fujiu, Project Associate Professor, and Dr. Issei Komuro, Professor in the Department of Cardiovascular Medicine at the University of Tokyo Hospital, Fujitsu has been engaged in research and development utilizing AI to detect heart disease from electrocardiogram data, leveraging Fujitsu’s proprietary waveform analysis technology, TDA (topological data analysis) (5). Going forward, the company will work to verify the effectiveness of AI in actual medical practice to estimate the presence or absence of abnormalities in cardiac function from electrocardiogram data, and will start clinical research at the University of Tokyo Hospital.
Clinical Research Overview
Based on the electrocardiogram data of patients examined at the University of Tokyo Hospital, Fujitsu and the University of Tokyo Hospital will verify the effectiveness of detection of abnormal heart movement by AI.