FX has completed the enrollment for their Investigational Device Exemption [IDE] study [G180100] on the Easytech Reversed® Stemless for reverse total shoulder arthroplasty becoming the first-and-only to do so.
“This is not only a major milestone for FX, but for the next generation of stemless shoulder arthroplasty and the way we think about reverse total shoulder arthroplasty. As a small company, we are excited and humbled to lead the way and be the first to complete this historic study that may bring a novel device to the U.S. market which has already seen over 2000 implanted in Europe since 2015,” said Baptiste Martin, CEO of FX Shoulder USA.
The study, that began in December of 2018 and concluded enrollment, consisted of (90) subjects completed across (7) sites.
“It has been a real pleasure over the last couple of years to participate in this IDE to offer patients a novel solution and to see very favorable outcomes for my patients. Stemless shoulder replacement is here to stay, and it was great being part of the trailblazing group to bring it to the U.S.,” said Michael Bradley, MD, MBA, MS of Ortho Rhode Island in Wakefield, RI.
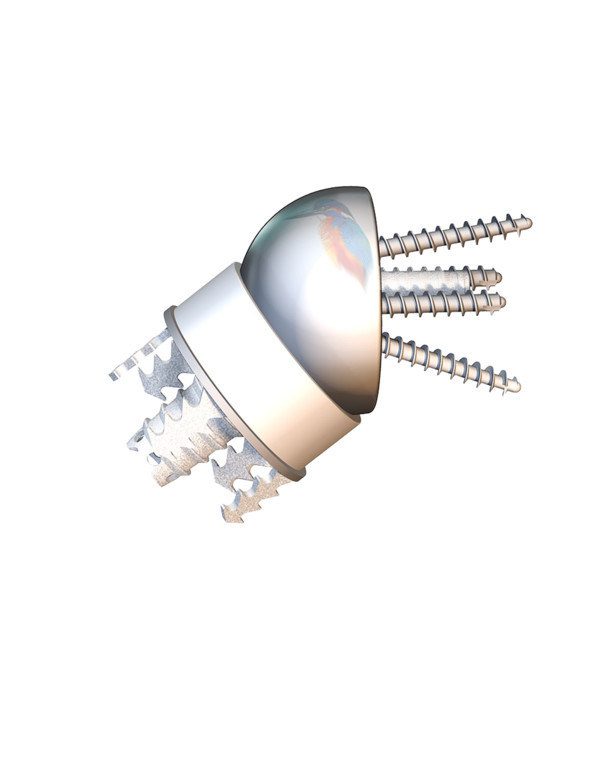
The Easytech Reversed® Stemless features peripheral fixation of the humeral component that is designed to be more bone sparing as compared to the traditional stemmed devices and its unique-to-market design for improved fixation to withstand the forces of reverse total shoulder arthroplasty.
The Easytech Reversed® anchor base is designed to fit peripherally just inside the cortical bone with optimal bone quality for fixation. It is the first-and-only stemless on the market with peripheral fixation which has been in use in the European market since 2015.
“The Easytech Reversed® is a unique stemless solution for reverse TSA to provide patients with an anatomically centered humeral articulating component that is bone sparing and has excellent fixation,” said Howard W. Harris, MD of Southlake, TX.
FX Shoulder USA, Inc. is based in Dallas, TX and is the direct provider of FX Solutions shoulder replacement devices in the U.S. FX Shoulder USA, Inc., founded in January 2018, focuses exclusively on shoulder arthroplasty. For additional information, please visit www.fxshoulder.com.
CAUTION: Investigational Device. Limited by Federal (or United States) Law to investigational use. (21 CFR812.5(a)) FDA: NCT 03806842.