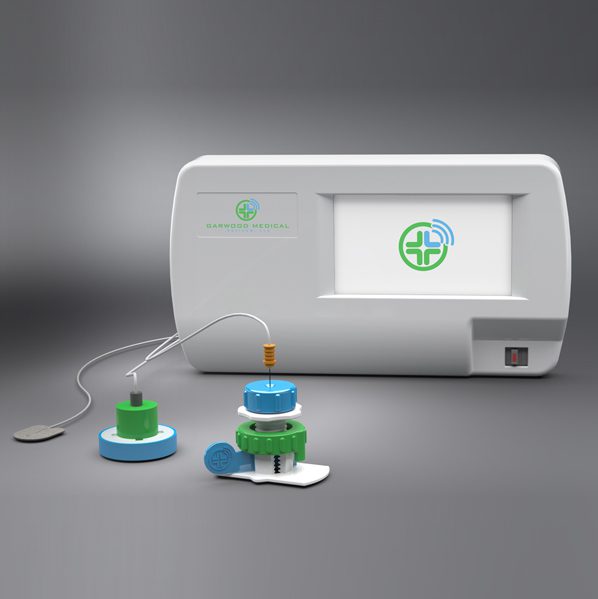
Garwood Medical Devices, LLC, an emerging Buffalo-based company developing groundbreaking BioPrax™ technology to treat antibiotic-resistant bacterial biofilm infections associated with metallic orthopaedic implants, announced today its Series C round of accredited-investor funding has closed at $4 million, as projected.
The third round of financing, which began last September, attracted institutional investment from groups such as The WNY Impact Investment Fund and the Murray family, former owners of UC Coatings, along with global investors in the orthopaedics/health care space.
Garwood Medical has now raised $11.4 million, including $3.8 million in the Series B round, which closed October 2019 and was over-subscribed by $800,000. The company raised an initial $3.6 million during the company’s Series A financing round, which closed in September 2016. Funds raised will continue to support pre-clinical testing.
“We are very pleased with this continued success in raising much-needed capital and attracting interested partners,” said Wayne Bacon, president & CEO of Garwood Medical. “Our enthusiasm is further bolstered by the fact that 90 percent of our A- and B-round investors reinvested, taking 60 percent of the C round.”
Bacon said the three rounds of funding have been supported by individuals, including many orthopaedic surgeons and medical professionals, who understand the tremendous promise and value proposition the company provides.
Garwood Medical’s BioPrax™ device is currently under investigation to study the elimination of biofilm infections on prosthetic knee implants during early intervention procedures and used alongside the current standard of care.
BioPrax™ was accepted into the FDA’s Breakthrough Device Program in October 2019, an important milestone on the path to market acceptance and widespread usage among orthopaedic surgeons and hospitals, anticipated in 2025.