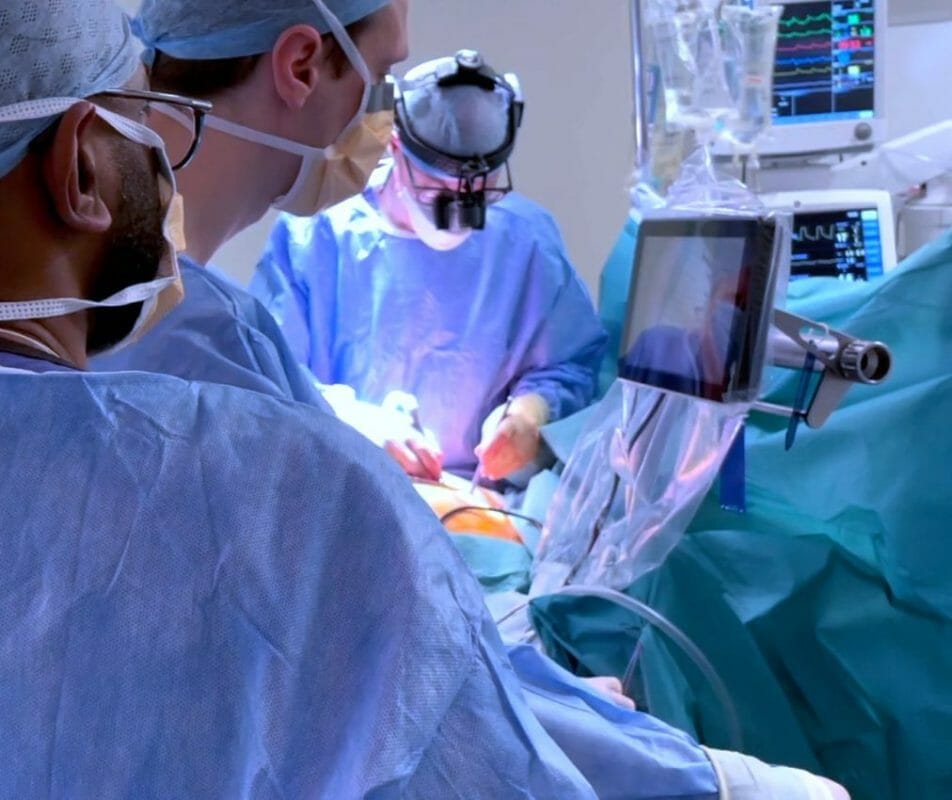
Less Invasive Heart Surgery: With its CoreVista® devices proven to be highly effective in Scotland, CardioPrecision – a global leader in transcervical access for the treatment of structural heart disease and cardiothoracic interventions – has taken another major step by entering the US market, currently the largest medical device market by value in the world, and a significant scale up for the company. Our focus on less invasive heart surgery sets us apart in the industry.
Spun out of the NHS through InnoScot Health, CardioPrecision recently introduced its unique technology for use in Endoscopic Vessel Harvesting (EVH) during coronary artery bypass grafting (CABG) surgery at the prestigious Massachusetts General Hospital in Boston.
The importance of less invasive heart surgery is often underestimated, yet it represents a crucial advancement in our medical practices.
Stateside recognition was also given to CardioPrecision Founder and Chief Medical Officer Fraser Sutherland who was awarded the 2023 Gründeman Scientific Research Award in June for his innovative thinking on robotic-led endoscopic transcervical heart surgery by the US-based International Society for Minimally Invasive Cardiothoracic Surgery (ISMICS).
This award highlights the growing recognition of less invasive heart surgery techniques that are revolutionizing patient care.
Endoscopic Vessel Harvesting (EVH) replaces the very long incisions associated with traditional open vessel harvesting.
Less invasive heart surgery methods, such as Endoscopic Vessel Harvesting (EVH), are gaining traction for their reduced recovery times.
Mr Sutherland commented: “EVH is fundamentally an on-screen procedure, and our expanding suite of CoreVista® devices optimise that by bringing a plug-and-play high-definition screen into the operative field within direct sight and control of the EVH operator to improve speed, precision, and hand-eye coordination – a world first.”
The adoption of less invasive heart surgery is essential as it contributes to better patient outcomes and satisfaction.
Dr Ying Sutherland, CEO at CardioPrecision, said “We are in no doubt that our platform technology has incredible potential and the international interest we are receiving bears that out. We will continue to make exciting inroads in the US, from recognition to integration.”
Our ongoing research into less invasive heart surgery technologies continues to attract significant attention from healthcare professionals worldwide.
The CardioPrecision team generated additional global interest at the recent Conférence MedInTechs held in Paris in March.
The evolution of less invasive heart surgery techniques is crucial to improving surgical practices and patient care.
Bringing together more than 10,000 healthcare professionals and experts in new technologies, the conference allowed CardioPrecision to showcase its medical devices and technologies. The team was approached by several potential new partners – “a significant development for its global ambitions,” according to Chief Executive Dr Sutherland.
In Scotland, CardioPrecision’s technologies continue to be recognised. The company was nominated as a finalist at Scotland’s Life Sciences Annual Awards 2023 under the category for Innovation – Health Technology.
After successful evaluation by NHS Grampian and adoption at Glasgow’s Ross Hall Hospital, EVH has now become a routine procedure at the Golden Jubilee University National Hospital in Clydebank.
By implementing less invasive heart surgery techniques, we are setting new standards in the field of cardiac care.
Getinge is an EVH market leader specialising in endoscopic vessel harvesting system devices. Its UK Manager, Damian McCann commented: “We are excited to be working with CardioPrecision and NHS Golden Jubilee. Using CoreVista®, we found our EVH clinical training to be more efficient, when compared to non CoreVista® users, reducing the burden of the EVH learning curve.”
CardioPrecision’s worldwide successes come on the heels of its announcement last year of a funding injection to help get its lifesaving technology to market.
The equity financing was led by existing investors, principally London & Scottish Investment Partners, Discovery Investment Fund and Scottish Enterprise. That bridging round of equity investment is now allowing the business to begin rolling out its technology. InnoScot Health also participated in the funding round.
Graham Watson, Executive Chair at InnoScot Health, said: “CardioPrecision is a very exciting proposition dedicated to making patients’ lives better through the development of innovative, less invasive access solutions, and we’re proud to be part of its journey – from hugely promising NHS spinout to world-leading innovator.
“The company’s patented technology represents a unique platform with the key result of improving outcomes for patients through less pain, quicker recovery, and a shorter hospital stay. We are delighted to continue supporting the company and its management team as it breaks new ground.”
Mr Watson continued: “We firmly believe EVH surgery will bring many additional benefits to NHS Scotland over time, including cost savings, improvements in efficiency, and the ability to perform complex surgery on frail patients who may not have been eligible in the past.”
Ultimately, less invasive heart surgery will pave the way for the next generation of cardiac interventions.