Editor: What To Know
- The device is packaged ready-to-use in a syringe, can be prepped tableside by the clinician in about 30 seconds, and may be delivered through standard microcatheters (no complex mixing systems or special delivery catheters are necessary).
- GPX technology is a low viscosity, aqueous-based solution in a syringe that solidifies into a durable embolus upon delivery without polymerization or dimethyl-sulfoxide (DMSO) precipitation.
- “Treating a tumor with multiple feeding vessels in a controlled, thorough manner without risk of catheter entrapment can improve patient care and minimize the need for follow-on procedures,” said Libble Ginster, CEO of Fluidx Medical Technology.
Fluidx Medical’s GPX Embolic Device was used to effectively devascularize a large tumor with multiple feeding vessels as part of a multi-center clinical trial.
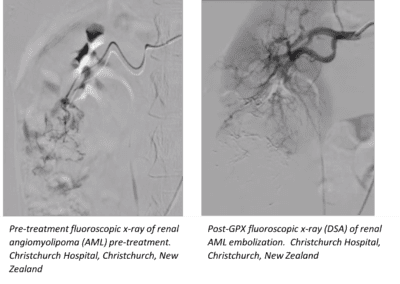 “This could have been a challenging case since it involved a large renal tumor fed by small, low flow, tortuous vessels. We were able to use our standard embolic microcatheter to deliver GPX in a highly controlled fashion. Since we were not worried about catheter entrapment, we could take our time and ensure that we occluded all targeted vessels completely. GPX flowed distally very well, completely filling the targeted region,” said Martin Krauss, M.D., Head of Interventional Radiology, Christchurch Hospital,
“This could have been a challenging case since it involved a large renal tumor fed by small, low flow, tortuous vessels. We were able to use our standard embolic microcatheter to deliver GPX in a highly controlled fashion. Since we were not worried about catheter entrapment, we could take our time and ensure that we occluded all targeted vessels completely. GPX flowed distally very well, completely filling the targeted region,” said Martin Krauss, M.D., Head of Interventional Radiology, Christchurch Hospital,
Christchurch, New Zealand. “Based on our case experiences, GPX is a great product for effectively filling distal vasculature.”
The GPX Embolic Device is an innovative embolic designed for simple preparation and controlled delivery. The device is packaged ready-to-use in a syringe, can be prepped tableside by the clinician in about 30 seconds, and may be delivered through standard microcatheters (no complex mixing systems or special delivery catheters are necessary).*
GPX technology is a low viscosity, aqueous-based solution in a syringe that solidifies into a durable embolus upon delivery without polymerization or dimethyl-sulfoxide (DMSO) precipitation. GPX is designed to occlude blood vessels independent of a patient’s coagulation situation.*
“Treating a tumor with multiple feeding vessels in a controlled, thorough manner without risk of catheter entrapment can improve patient care and minimize the need for follow-on procedures,” said Libble Ginster, CEO of Fluidx Medical Technology. “GPX demonstrates improved control and precision. GPX does not require 20+ minutes of preparation time or the clinician to use a special catheter system. The simplicity of GPX preparation makes real-time clinical decision-making possible. We continue to be excited about the GPX portfolio and its future in advancing cancer care.”
Fluidx Medical Technology is a Salt Lake City, Utah based company focused on developing the GPX Embolic Device and other innovative medical technologies.
The GPX Embolic Device is under development and does not have marketing clearance or approval in any market at this time. For investigational use (in New Zealand) only.
*Data on file. Fluidx Medical.

