Healionics Corporation reports the financing round was led by Keiretsu Capital. The company also announced the conversion to equity of $5.2M in previously outstanding notes and interest.
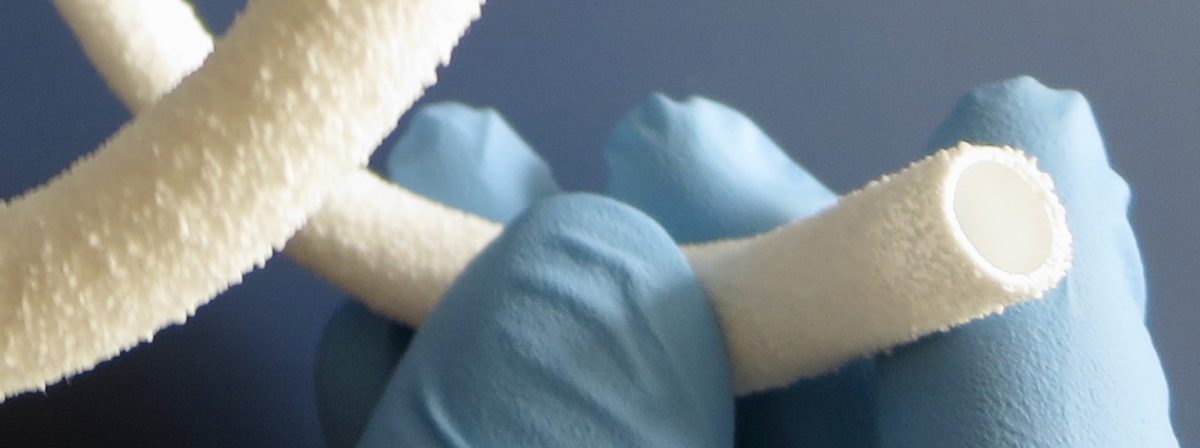
Healionics is preparing to commercialize its innovative STARgraft vascular graft, designed to provide a safer and more reliable means to access the bloodstream for dialysis in patients with kidney failure. An initial human study was completed last year, and a follow-on human study is now in progress. The financing will be used to complete this study and obtain FDA market clearance.
“We appreciate the strong support in this round by existing shareholders and welcome the participation by several new angel and venture investor groups,” said Mike Connolly, Chief Executive Officer of Healionics. “We are targeting early next year for the commercial launch of STARgraft. This novel synthetic blood vessel, based on our proprietary STAR biomaterial technology, has the potential to improve the lives of dialysis patients by reducing the frequency & severity of infections and the frequency of interventions required to maintain dialysis access.”
More than 500,000 people in the United States suffer from kidney failure and require frequent dialysis to filter waste from their blood. Current methods of creating and maintaining regular bloodstream access for dialysis are unreliable and account for a significant portion of the $50 billion spent each year on U.S. kidney-failure patients. A vascular graft (synthetic blood vessel) is often implanted to create an access site with sufficient flow rate for dialysis, but conventional grafts can develop life-threatening infections and frequently develop blockages that require expensive interventions.