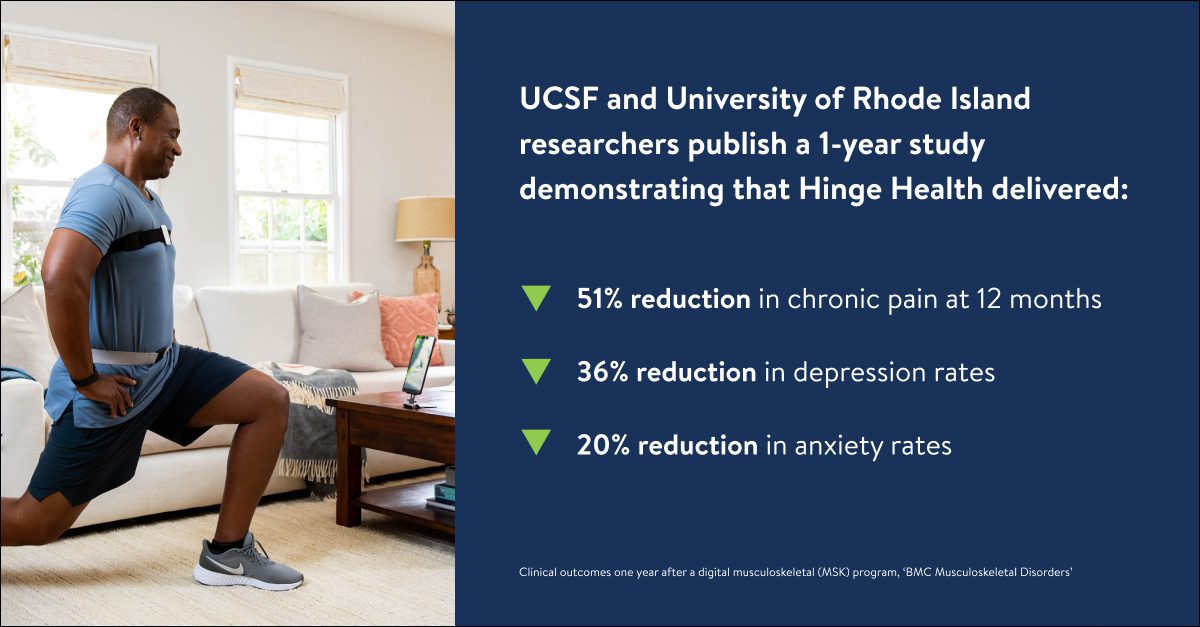
Hinge Health announced the results of the first 12-month outcome study demonstrating the long-term benefits of digital care for chronic musculoskeletal (MSK) pain. The peer-reviewed study, published in BMC Musculoskeletal Disorders, showed that pain was reduced by more than half (51%) and sustained over 12 months among Hinge Health participants. Additionally, depression, anxiety, and medical utilization rates decreased after a year.
Chronic musculoskeletal (MSK) pain is a leading cause of disability and healthcare costs in the United States – accounting for up to 75% of an employer’s MSK costs. Rates of osteoarthritis, back and neck pain, and other MSK disorders in the United States are among the highest in the world, with 1 in 2 reporting an MSK condition. The study provides important evidence to employers and health plans looking to modernize their benefits program with an MSK solution that has delivered long-term outcome improvements.
Researchers from the University of California, San Francisco and the University of Rhode Island, compared Hinge Health’s digital MSK program participants with nonparticipants (n = 2570). The study examined pain, function, depression, and anxiety at 3, 6, and 12 months and health care use at 12 months. The intervention group engaged in a digital MSK program that included exercise therapy, education, and health coaching for at least 3 months.
Key outcomes demonstrating the success of Hinge Health’s approach include:
Long-term pain improvement: On average, Hinge Health program participants reported 51% pain reduction sustained over 12 months. 72% of the program participants had clinically meaningful pain reduction at 12 months.
Lowered rates of depression and anxiety: Hinge Health participants were 36% less likely to report moderate to severe depression at 12 months, and 20% less likely to report moderate to severe anxiety.
Reduced medical utilization: Over the course of the year, the researchers also found that Hinge Health participants were 17% less likely than non-participants to seek additional healthcare services, such as office visits, surgeries, or imaging.
“Digital health studies have traditionally looked at short-term benefits where members have recently completed their pain management program or post-operative patients where their pain is already expected to go down,” said Dr. Jeff Krauss, chief medical officer, Hinge Health. “We’re excited to share the industry’s first peer-reviewed study that measures chronic pain improvement at 12 months and demonstrates long-term benefits of a digital MSK program that uniquely combines clinical care teams with advanced technology and data. The study is significant since it demonstrates both improved clinical outcomes and reduced healthcare utilization over the long-term.”
“What I really like about Hinge Health is that they pioneered digital MSK care for enterprises and since then led the way with research and clinical studies,” said Matthew Harmon, vice president of Benefits, Compensation & HR Systems at AutoZone. “We’re excited to see new data on sustained improvement among Hinge Health program participants. It further validates our commitment to expand high-quality MSK care for our members.”
“This exciting research examining outcomes data from Hinge Health’s promising digital MSK program provides new and potentially transformative insights on the long-term effectiveness of holistic digital care in treating chronic pain,” said Jeannie Bailey, Ph.D., senior study author and assistant professor of Orthopedic Surgery, University of California, San Francisco.
This 1-year outcomes study builds on previously published studies. Hinge Health has consistently demonstrated reductions in MSK pain and improvements in mental health through clinically-validated studies that include a large-scale 10,000-participant study, three randomized controlled trials, and the industry’s only study showing the impact of a digital MSK program on an older adult population.