In a groundbreaking study, Hoag Hospital, a leading not-for-profit regional health care delivery network in Orange County, California, presented the results of a two-year analysis at the 2023 American Association of Orthopedic Executives (AAOE) conference. The study demonstrated that the Luna in-home outpatient physical therapy care model reduces costs by 52% compared with traditional home health for total knee and hip replacements.
Hoag Orthopedic Institute conducted the study between 2021 and 2023 in collaboration with Luna, the leading provider of in-home outpatient physical therapy, to create a scalable solution aimed at reducing per episode care costs through the use of in-home outpatient physical therapy in lieu of traditional home health services. The complete study can be viewed here.
As healthcare spending continues to rise, the American Academy of Orthopaedic Surgeons forecast a substantial increase in total knee replacement or total hip replacement surgery, with projected growth rates of up to 171% and 189% respectively (source) by 2030.
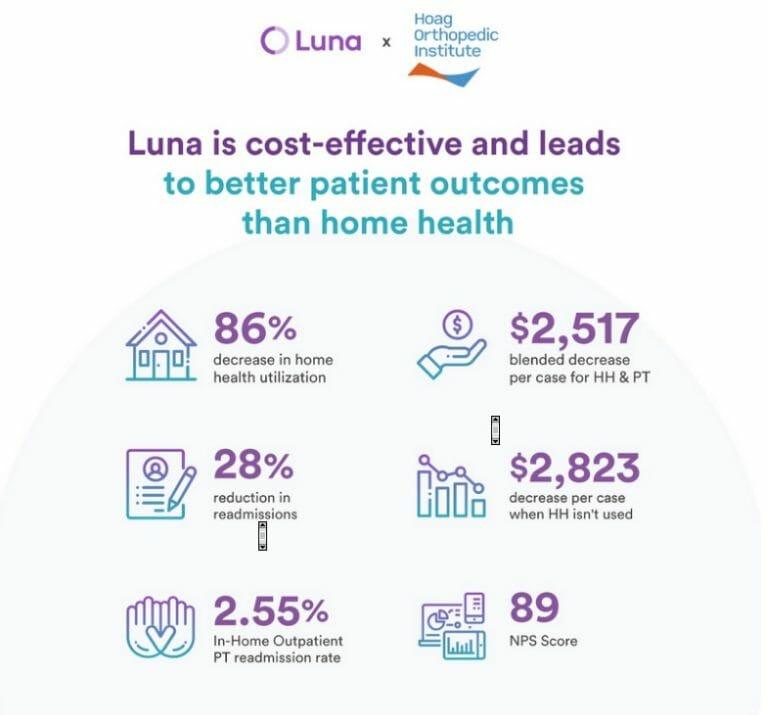
The Hoag Orthopedic Institute study showed that with the Luna care model, participating surgeons reduced their home health utilization by 86%, resulting in decreased blended costs of $2,517 per case. Patient outcomes also improved as readmission rates were reduced by 28%, compared with patients that received the traditional home health option.
Patients also demonstrated clinically important changes in pain and patient-specific functional scale scores of 66% and 76% respectively, and rated Luna with a world-class net promoter score of 89.
“Luna’s care model is cost-effective and leads to better patient outcomes when compared with traditional home health, and is revolutionizing healthcare delivery nationwide. By making high-quality care more accessible to patients, Luna can provide postoperative total hip replacement and total knee replacement patients with a more efficient alternative to traditional home health services,” said Palak Shah, Co-Founder and Head of Clinical Operations at Luna.
At Hoag, overutilization of home health visits was also a challenge, with patients utilizing an average of eight visits of home health prior to continuing care at an outpatient facility. While a typical course of care at Luna consists of completing the entire care plan with a Luna therapist (all billed under outpatient rates, and delivered to the home), Hoag had patients complete the initial four to six treatment sessions with Luna prior to transitioning to an outpatient facility.
Patients completed four visits for total hip replacements and six visits for total knee replacements with the Luna therapist. During care at Luna, patients were monitored for visit utilization, post-surgical complications, readmissions, and adverse outcomes.
Innovative healthcare organizations are exploring cost-effective ways of delivering care while continuing to improve patient outcomes, and, to date, Luna has collaborated with Providence, Emory Healthcare, Intermountain Healthcare, UCLA Health and Scripps Health, among others.
Luna currently operates across 48 markets in 27 states, and has treated 35,000+ patients in their homes. In 2022, Luna became the fastest growing physical therapy clinic nationally with 6,183 percent growth in visits during the four consecutive years since it was founded in 2018. This is higher than any reported growth during the same time frame from any physical therapy clinic group.