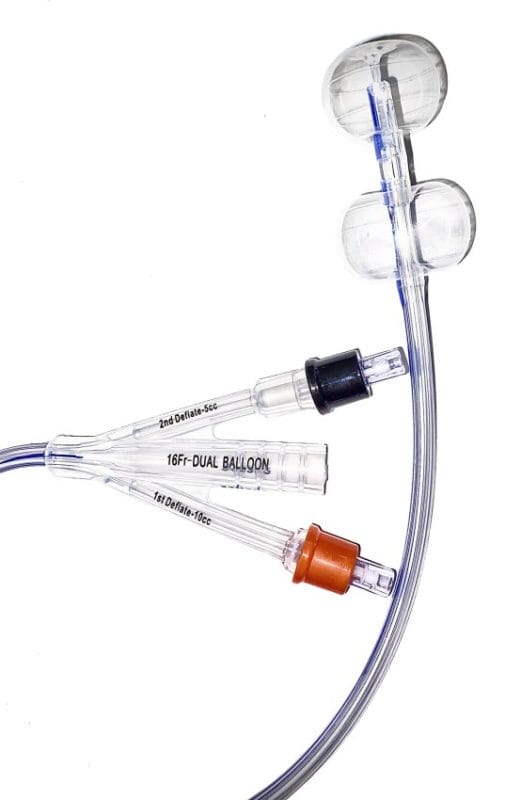
HR Pharmaceuticals, Inc. (HRP) has obtained exclusive commercialization rights for Poiesis Medical LLC’s Dual Balloon Catheter (Duette™) in North America, further enhancing HRP’s urological portfolio and addressing adverse events related to Foley catheterization for specific patient populations.
The Dual Balloon Catheter has demonstrated significant benefits compared to traditional Foley catheters, leading to better patient outcomes. Specifically, we see a great opportunity for patients requiring indwelling catheterization greater than five days. This product will become an important part of our broader urology strategy over the next 12 months,” states Colby Wiesman, President of HR Pharmaceuticals, Inc.
“The Dual Balloon Catheter technology, a brainchild of my father, Dr. Bruce Wiita, a practicing urologist and decorated Vietnam veteran, was born out of a genuine desire to improve the quality of life for patients enduring prolonged catheterization and to alleviate the strains it places on the healthcare system,” says Gregory Wiita, CEO and Founder at Poiesis Medical LLC. “We are excited about this new chapter with HR Pharmaceuticals and the potential of the technology to make a meaningful impact in patient care.”
HRP will begin processing North American orders for the Duette Dual Balloon Catheters starting January 2, 2024. Duette is available at most national distributors, including Cardinal Health, McKesson, Medline, and Owens & Minor.