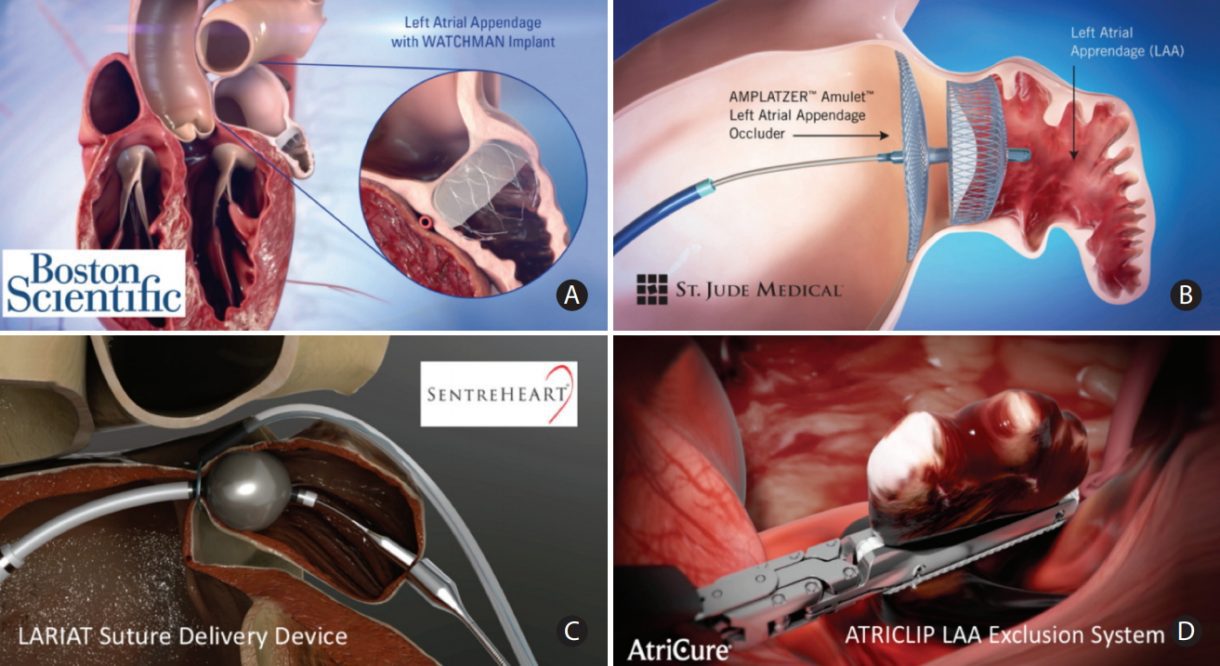
During the Heart Rhythm Scientific Sessions this week, Boston Scientific announced positive LBCT data underscoring the safety and efficacy of multi-site pacing (MSP) over conventional cardiac resynchronization therapy (CRT), as well as poster presentations on real-world use of the WATCHMAN Left Atrial Appendage Closure (LAAC) device and next-generation WATCHMAN FLX LAAC device as alternatives to oral anticoagulation therapy for stroke risk reduction in patients with non-valvular atrial fibrillation.
Key findings from the studies include:
Data demonstrated that MSP with RESONATE™ X4 CRT-D devices is safe and effective when used in non-responders to conventional CRT, and showed that MSP can convert non-responders to responders with minimal impact to battery life of the CRT-D devices. The study had an initial responder rate to CRT therapy of 74% and exceeded pre-determined performance and safety goals:
o At 12 months, 78 patients met the effectiveness endpoint analysis requirements and demonstrated a response rate to MSP of 51.3% (40/78), compared to a predefined goal of 5%.
o A 99% complication free rate compared to a predefined goal of 90%.
FLXibility Post-Approval Study
- The first patient follow-up visit data from the European FLXibility registry with the next-generation WATCHMAN FLX device demonstrated excellent procedural success rates in everyday clinical practice.
o This included high effective LAA closure rates (87.6% complete seal, 12.4% residual blood flow size ≤ 5mm) and low short-term complications through 120 days, including 0.7% all-stroke, 1% pericardial effusion, 0.3% device embolization and no cases of systemic embolism.
o These positive results are similar to those observed in the PINNACLE FLX pivotal approval trial, for which 2 -year results were just announced.
NESTed Post Approval Study
- Two-year data from the NESTed Post-Approval Study with the WATCHMAN device, stemming from the NCDR LAAO Registry, demonstrated low rates of stroke and systemic embolism.
o The study met its primary safety endpoint (defined as all-cause death, ischemic stroke, systemic embolism or procedure-related events requiring intervention within 7 days or hospital discharge) with a rate of 1.4%; and second primary efficacy endpoint (defined as 2-year ischemic stroke and systemic embolism) with a rate of 3.0%.
o The first primary efficacy endpoint (defined as 2-year all-cause death, hemorrhagic stroke, ischemic stroke and systemic embolism) exceeded the performance goal at 17.1%, (PG was <15%), but was driven by non-cardiovascular mortality in this older, high-risk population.