November 2, 2020
IAI (Intelligent Actuator Incorporated), a leading robotics provider headquartered in Shizuoka, Japan, would like to introduce their CRS 6-axis Cartesian robot.
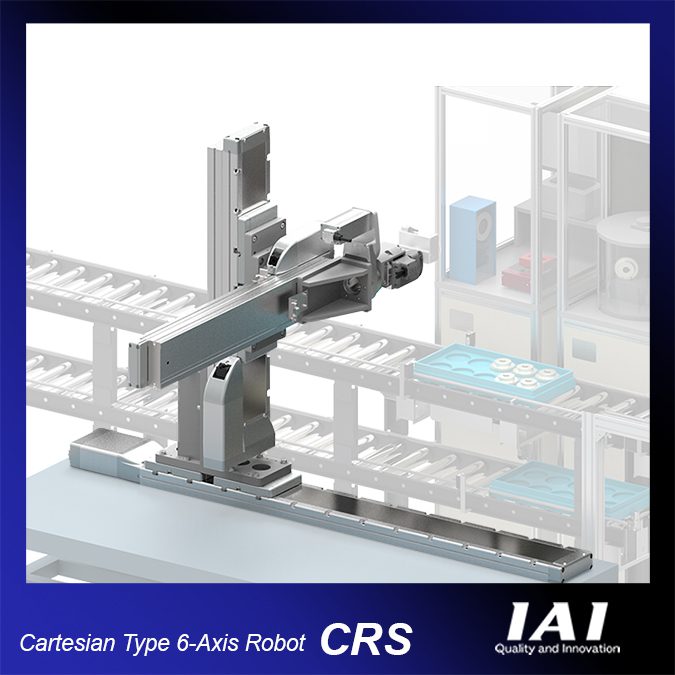
IAI reports this 6-axis robot design is excellent for high speed and accurate pick-and-place operations. With 6 degrees of freedom combining 3 Cartesian and 3 rotational axes, operations with a high level of freedom are possible, including rotation and turning.
The CRS has many variations available. You can select the optimal system based on your payload, stroke travel, and installation space. There are combinations that are ideal for three-dimensional movements within a specified work envelope and combinations that are perfect for accessing difficult to reach locations such as loading and unloading machines or storage shelves. All axes are equipped with battery-less absolute encoders as a standard feature.
“IAI is proud to be in the forefront of technology to assist the medical community in the battle against Covid-19 and any future health crisis,” says Kaz Haruna, National Sales Manager for IAI America. The current pandemic creates an urgent need for readily available multiple testing and diagnostic tools worldwide.
IAI is actively assisting the medical community by providing Cartesian and SCARA robots for infectious disease testing, molecular diagnostics, tissue and blood sampling, genomic research, cancer research, pharmaceuticals, and medical device production. It is IAI’s hope that our new 6-axis CRS robots will help the medical/pharmaceutical industries move ahead with advanced innovation.