Fluidx Medical Technology announced that a next-generation, doxorubicin-loaded GPX Embolic Device was featured at the recent Symposium on Clinical Interventional Oncology (CIO) Conference highlighting the technology’s potential for oncology drug delivery.
“GPX is easy to prepare, deliver, and control,” said Ryan O’Hara, M.D., Interventional Oncologist, University of Utah, Salt Lake City, Utah. “The initial results of GPX as a drug-loadable oncology solution are very promising. GPX would be the first loadable liquid embolic designed for tumor applications.”
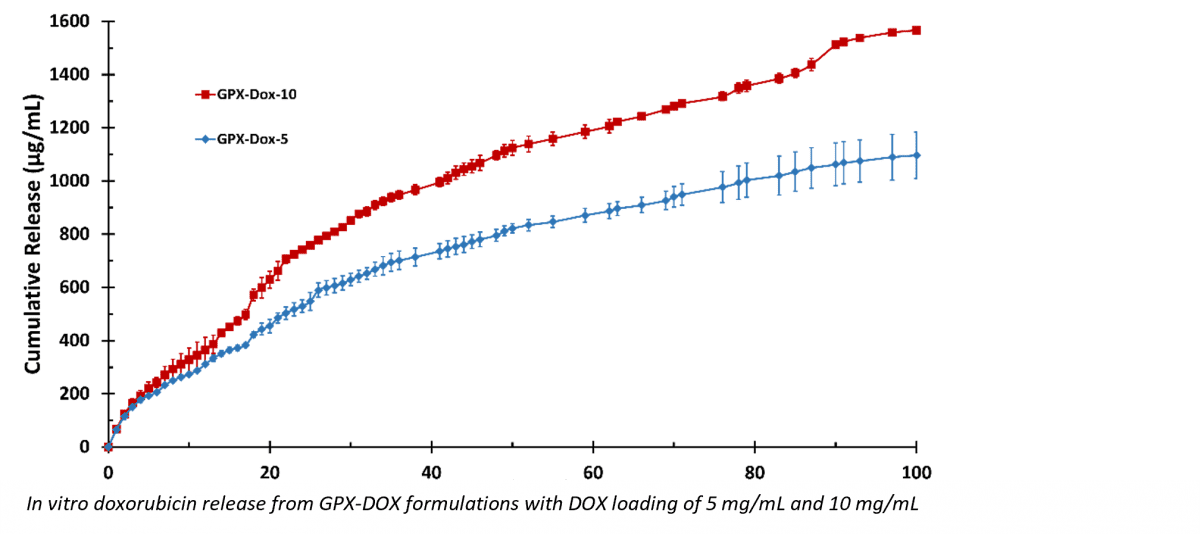
Study results presented at CIO included an in vitro characterization of the loading and drug release profile of the GPX Embolic Material loaded with doxorubicin (GPX-DOX). GPX-DOX provided a zero-order release profile for 5 weeks and sustained release out to 100 days. The long linear release profile is desirable in drug delivery, as a constant amount of drug is delivered regardless of the concentration within the carrier. These properties are unique in the field of liquid embolics.
The GPX Embolic Device is an innovative embolic designed for simple preparation and quick material delivery. GPX technology is a low viscosity, aqueous-based solution in a syringe that solidifies into a durable embolus upon delivery without polymerization or dimethyl-sulfoxide (DMSO) precipitation. GPX is designed to occlude blood vessels independent of a patient’s coagulation situation. The device is packaged ready-to-use in a syringe, requires less than 30 seconds of tableside preparation by the clinician, and may be delivered through standard catheters or small microcatheters (no complex mixing systems or special delivery catheters are necessary). *
“We have not seen other liquid embolic devices that have demonstrated the ability to load and release oncology agents in a sustained manner,” said Libble Ginster, CEO of Fluidx Medical Technology. “The Fluidx team is excited about the future of GPX technology and inspired by bringing new innovations to cancer patient care.”
Fluidx Medical Technology is a Salt Lake City, Utah-based company focused on developing the GPX Embolic Device and other innovative medical technologies.
The GPX Embolic Device is under development and does not have marketing clearance or approval in any market at this time. For investigational use (in New Zealand) only. GPX-DOX Embolic Device is under development and does not have marketing clearance or approval in any market at this time.