Innoventric, a start-up company from Ness-Ziona, Israel, develops the unique Trillium™ technology for the treatment of Tricuspid Valve Regurgitation. The company was founded in 2017, and has rapidly progressed. Innoventric has performed over 10 implantations of the promising Trillium™ technology as part of its European first in human clinical trial. The company holds a broad patent portfolio for the Trillium™ and additional Tricuspid technologies.
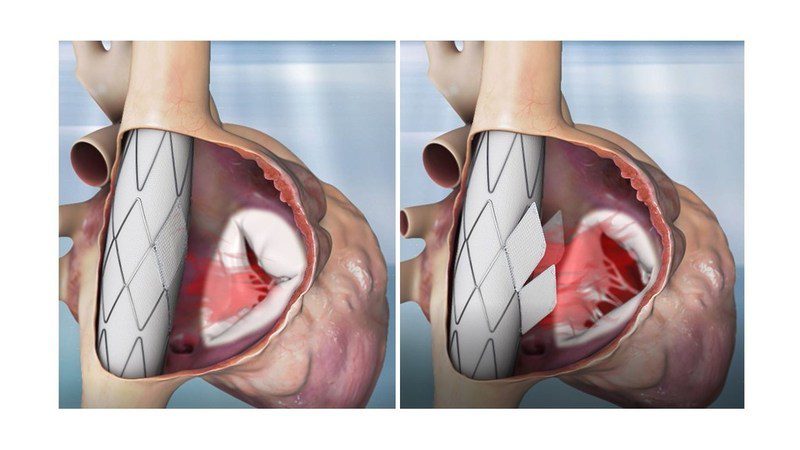
In the past 15 years, there have been many attempts to develop transcatheter tricuspid valve replacements. Despite intense efforts, there is no commercially available technology or a clear solution which has been widely adopted by the clinical community. The Trillium™ presents a unique, proprietary approach for functional tricuspid valve replacement. This approach easily overcomes the traditional valve replacement main barriers by avoiding the native tricuspid valve as a fixation site. Instead, the tubular and symmetric caval veins act as the fixation sites, allowing the Trillium™ to function along-side the failing native valve rather than replacing it.
Amir Danino, Founder and CEO, said: In the last decade structural heart interventions have become extremely complex and risky. This is manifested, among else, by the duration of these procedures and by their sub-optimal technical success rates.
The Trillium™ technology is extremely simple and effective, implantation takes less than 15 minutes to perform, and the technical success rate we see in our study is 100%. No aborted procedures, no migrations, no device fractures.
The physiological results are clear and immediate – abolition of backflow leads to a significant decrease in patients’ venous pressure. This improves patient renal and hepatic function, decreases peripheral edema and improves overall quality of life.
Rafi Benary, Co-founder and Head of Business Development, said: It is quite an exciting experience to be part of a company which has come so far in such a short time, and which has managed to bring such a differentiating technology to help a large and ailing population. It is easy to sense the excitement that our technology generates in the clinical community. We are confident that the Trillium™, as well as our emerging developments will provide Cardiologists with the right tools for treating their patients. Tricuspid disease is a large and growing area and we are confident in our ability to make an impact.
Innoventric was founded, by Mr. Amir Danino, who is also the inventor of the company’s technology, Mr. Rafi Benary and Dr. David Planer, MD, Head of Interventional Cardiology at Hadassah Medical Center and the company’s CMO.
Four years ago, the company raised its seed investment from two Leading Israeli MedTech venture capitals. Seed money successfully took the company and its novel technology from concept to clinical studies, proving the Trillium’s feasibility, safety and performance in the pre-clinical and clinical settings. In addition to the seed investment, Innoventric was acknowledged for four consecutive years by the Israeli Innovation Authority, providing the company with additional funds as well as a hallmark for its technology and capabilities.
Recently, the company secured $14M through its Series A financing, this includes the prestigious Horizon Europe Grant for the sum of €2.5M. The round was led by a US based investor joined by a new European investor and all previous round participants.
Horizon Europe is a 6-year program, initiated by the European Union in 2021. The program aims to identify the top 1-2% companies/institutions in Europe and additional countries (e.g., Israel) which have groundbreaking technologies with high potential to disrupt the market and scale-up. The winning companies receive a grant that is intended to support the company growth. Innoventric was among 4,000+ applicants for the first Horizon Europe submission and after a very long and competitive process was acknowledged among the top 65 (1.5%) technology companies. The company was granted with the maximal grant support of €2.5M.
Mr. Danino referred to the current investment round and said: we are delighted to complete this financing round and are honored by the vote of confidence from our investors. Not only that we were able to raise more than we initially required, we are happy to see our new investors, along with all previous investors, participate in this round.
Last but not least, we are extremely proud of our Horizon Europe achievement, and want to thank the European Commission for acknowledging our company and technology. This prestigious prize provides us with the highest seal of excellence granted by the European Union to a technological start-up.