Instylla, Inc., a privately held company developing next-generation liquid embolics for peripheral vascular embolotherapy, today announced the publication of resultsi from the first-in-human clinical trial of Embrace™ Hydrogel Embolic System (HES) for the treatment of hypervascular tumors, in the Journal of Vascular and Interventional Radiology (JVIR).
This prospective, single-arm multicenter study demonstrated that Embrace HES effectively embolized malignant and benign hypervascular tumors by blocking tumor blood supply with technical success and persistent embolizationi, as noted in imaging follow-up at 30-days, in all eight patients treated on this study.
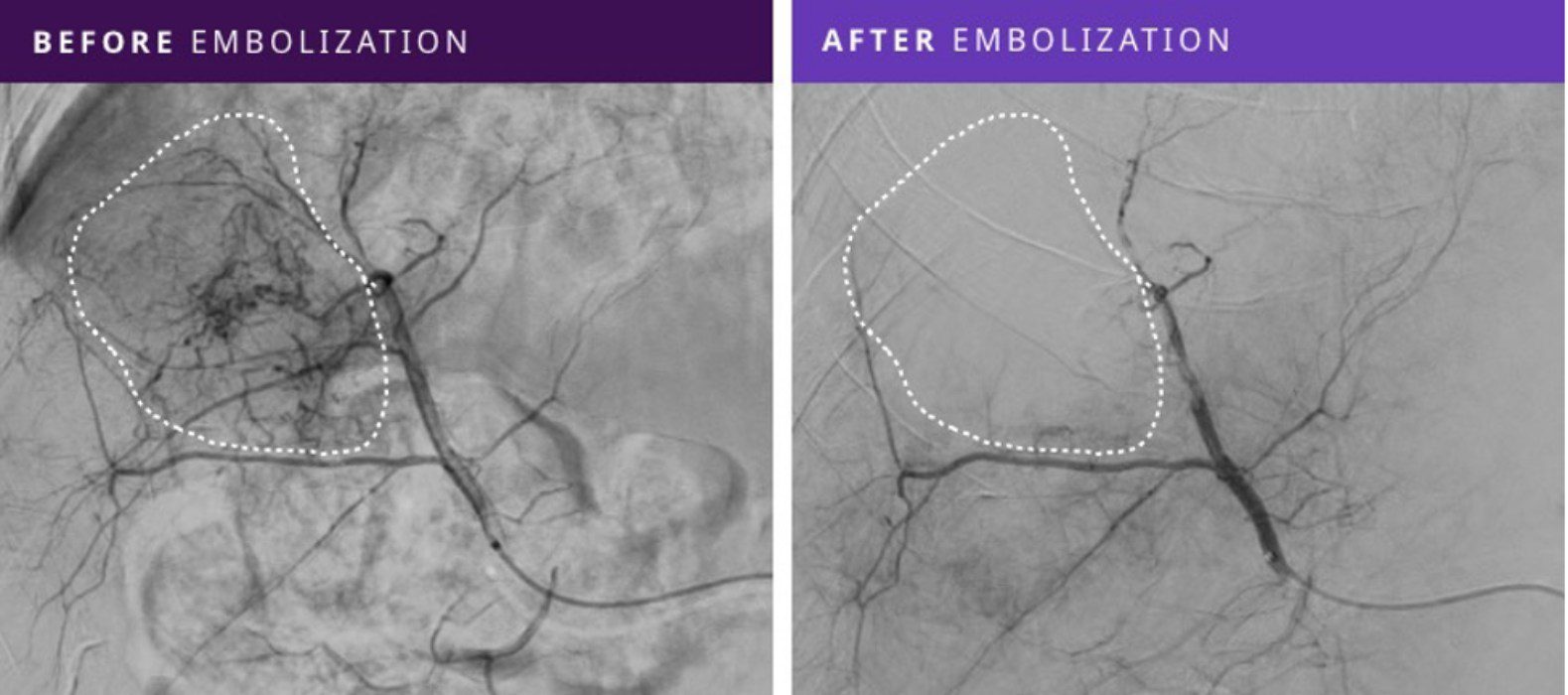
“This Phase I First-in-Human Study of Embrace HES in the embolization of a range of hypervascular tumors has shown promising results,” commented Dr. Gerard Goh, Head of Interventional Radiology at The Alfred Hospital and Associate Professor at Monash University, Melbourne, Australia, and lead author on the study. “Embrace HES holds great potential with its ease of use, technical success in all patients, and no tumor revascularization in the 30-day follow-up imaging.”
Ten embolizations were performed in 8 patients with a range of tumor types, including hepatocellular carcinoma (HCC), intrahepatic cholangiocarcinoma (ICC), and angiomyolipoma (AML). Tumor sizes ranged from 2.1 – 7.5 cm in size and were treated with Embrace HES volumes from 0.4 to 4.0 mL, with an average delivery time of 15 minutes.
Dr. Jee-Fu Huang, Professor, Department of Internal Medicine, Kaohsiung Medical University Hospital, Kaohsiung, Taiwan, added, “Taiwan has one of the highest age-adjusted incident rates of HCC in East Asia, which is also the second leading cause of cancer-related deaths. This novel liquid embolic, designed to achieve capillary level embolizationii, had favorable patient outcomes in the FIH trial, including HCC tumors. We are encouraged by these results. Our center is currently enrolling in the Instylla HES HVT pivotal study, so I look forward to continuing to assess Embrace HES in treating hypervascular tumors.”
About Embrace Hydrogel Embolic System:
Embrace HES is an investigational device intended to be used to embolize hypervascular tumors in vessels ≤ 5mm. Embrace HES consists of two low viscosity liquid precursors that solidify when simultaneously delivered into blood vessels, forming a soft hydrogel that fills the vessel lumens during the embolization procedure. The Embrace HES embolization uses no solvents, does not need sizing to the vessel diameter, and eliminates the possibility of catheter entrapment. Its main components are water and polyethylene glycol (PEG).