BetterLix
Amsety, a liver health solutions company announced the release of its new AI-powered app – BetterLix, a smart digital liver health coach. Developed by liver health experts and mathematicians, BetterLix utilizes the power of AI and the latest research on liver health to create personalized daily liver health plans. These plans encompass tailored lifestyle and diet recommendations to meet individual health needs. By providing guidance on liver health, BetterLix is designed to tackle the root causes of the liver disease epidemic: poor nutrition and unhealthy lifestyle.
Liver Disease in the U.S.
The launch of BetterLix comes at a critical time for liver patients. In last few decades, fatty liver disease has become an epidemic in the U.S. with now over 100 million Americans being affected by liver conditions, mostly fatty liver disease or also called metabolic dysfunction-associated fatty liver disease (MAFLD). Poor nutrition and a sedentary lifestyle are considered the major risk factors for developing fatty liver disease. Implementing lifestyle and diet changes, however, can prevent or even reverse MAFLD. With limited pharmacological treatments, the role of lifestyle and diet modifications is critical.
Despite an alarmingly high prevalence of fatty liver disease in the U.S., the availability of guidance on a liver-healthy lifestyle is insufficient. In Amsety´s survey conducted at The AASLD Liver Meeting in 2023, 72% liver health experts believe that “better nutrition guidelines” is the number one priority in lifestyle interventions. 92% of liver health experts also suggest the use of a digital tool that provides guidance on a liver-healthy lifestyle and nutrition to liver patients.
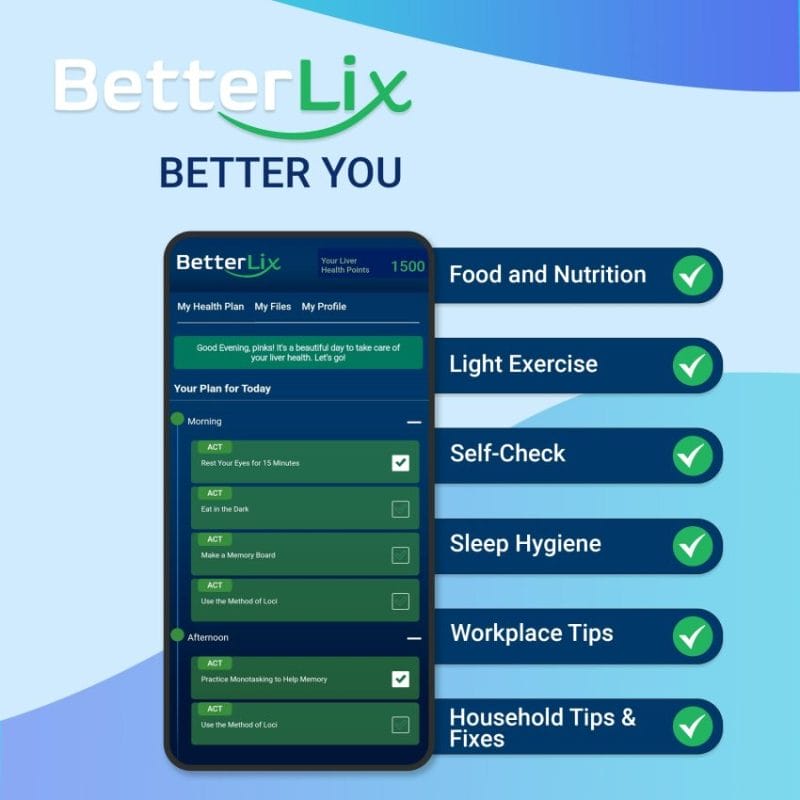
How does BetterLix work?
It generates easy and scientifically backed tasks that fit into daily routines, assisting liver patients in establishing a sustainable and long-term liver-healthy lifestyle. The tasks are tailored to the individual preferences and profile of each patient. The App makes it easy to follow a healthy lifestyle by providing instant feedback and progress tracking. BetterLix utilizes a comprehensive approach to liver disease and offers guidance on such areas as food and nutrition, exercise, sleep, workplace, mental health, and hydration.
For healthcare providers, BetterLix opens more efficient ways to reach and connect with liver patients.