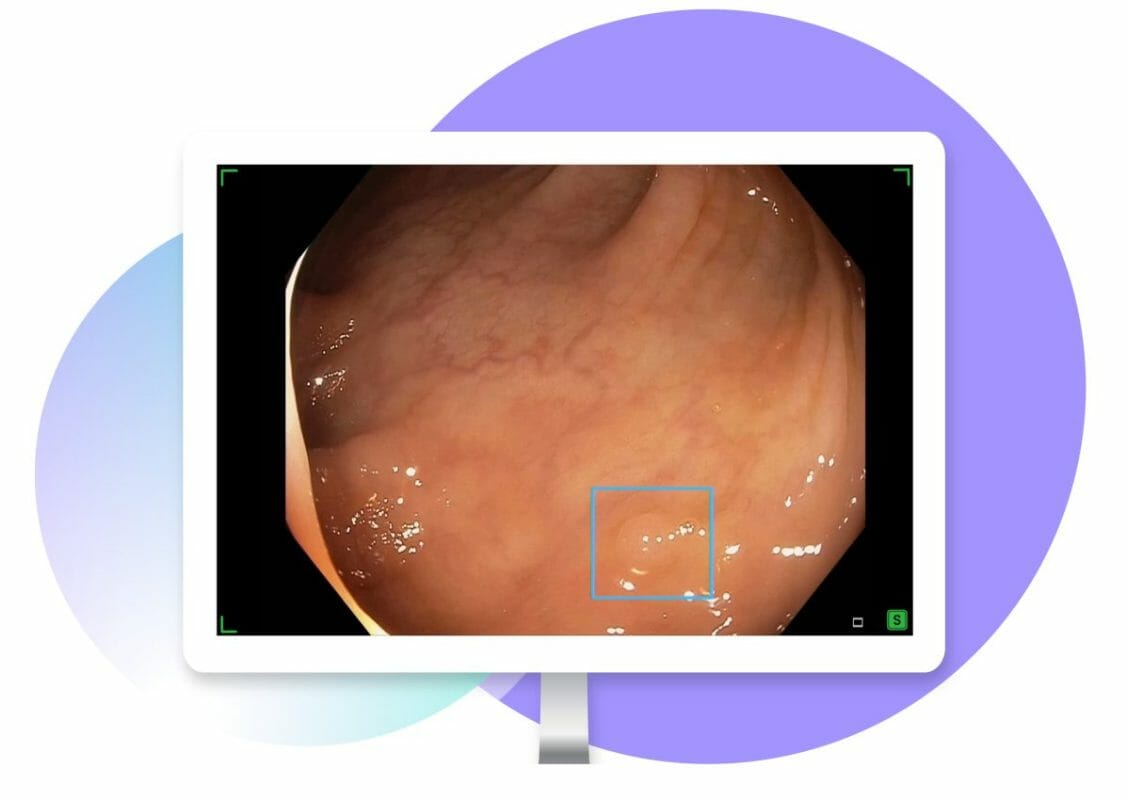
Iterative Health, a pioneer in precision-medicine technologies for gastroenterology, and Provation, the premier software and SaaS provider of clinical productivity and workflow automation solutions and a wholly-owned operating company of Fortive Corporation (NYSE: FTV), announced today the upcoming commercial availability of SKOUT, real-time artificial intelligence for polyp detection, in the second quarter of 2023. SKOUT is indicated as a computer-aided detection tool to assist qualified and trained endoscopists in identifying potential colorectal polyps during colonoscopy examinations in adult patients undergoing colorectal cancer screening or surveillance.
Colorectal cancer is the second leading cause of cancer-related deaths in the United States, and thorough colonoscopies are vital to preventing and diagnosing this condition. While current guidelines recommend an adenoma detection rate (ADR) of at least 25%4, detection rates among endoscopists range from 7-53%5 and polyp detection rates may decline by over 12% between morning and afternoon procedures. Artificial intelligence can help maintain a high-quality colonoscopy throughout the day.
Iterative Health developed SKOUT to help gastroenterologists detect more adenomas. In a randomized controlled trial published in Gastroenterology, SKOUT demonstrated a 27% relative increase in the detection of adenomas per colonoscopy (APC). Higher APC rates have been shown to lead to improved patient outcomes; a recent study showed that the incidence of colorectal cancer within three years of examination decreases with higher APC rates.
“We’re thrilled to announce that SKOUT will soon be available to gastroenterologists throughout the U.S.,” said Shrujal Baxi, MD, Chief Medical Officer at Iterative Health. “Adenoma detection can be enhanced, even among the best endoscopists, and the implementation of SKOUT provides an extra degree of surety for gastroenterologists that can have a positive impact on patient care.”
SKOUT, which is the only FDA-cleared device of its kind evaluated in a US-based clinical study population,* will serve gastroenterologists across the country by:
- Detecting more adenomas with real-time AI. SKOUT enhances procedure quality with increased adenoma detection, and reduces challenges in detecting certain polyp types, such as flat polyps and polyps in the proximal colon.
- Preserving existing workflows. SKOUT does not impact total procedure or withdrawal time and integrates seamlessly into existing clinical workflows. Designed with the gastroenterologist in mind, SKOUT features smart detection of endoscopic tools to keep the visual field clear during resection.
“SKOUT equips patients and providers with an extra degree of confidence that a high-quality colonoscopy was conducted,” said Aasma Shaukat, MD, MPH, Robert M. and Mary H. Glickman Professor of Medicine and Gastroenterology at NYU Grossman School of Medicine, and a primary investigator on the SKOUT registration study. “Computer-aided detection has the potential to raise the overall standard of gastroenterological care for patients.”
“We are excited that Iterative Health and Provation will jointly bring SKOUT to market as the next advancement in the field of gastroenterology,” said Ankush Kaul, President of Provation. “SKOUT will change the way care is delivered in the industry, and we are extremely enthusiastic about the results we believe it will have for patients.”