April 13, 2021
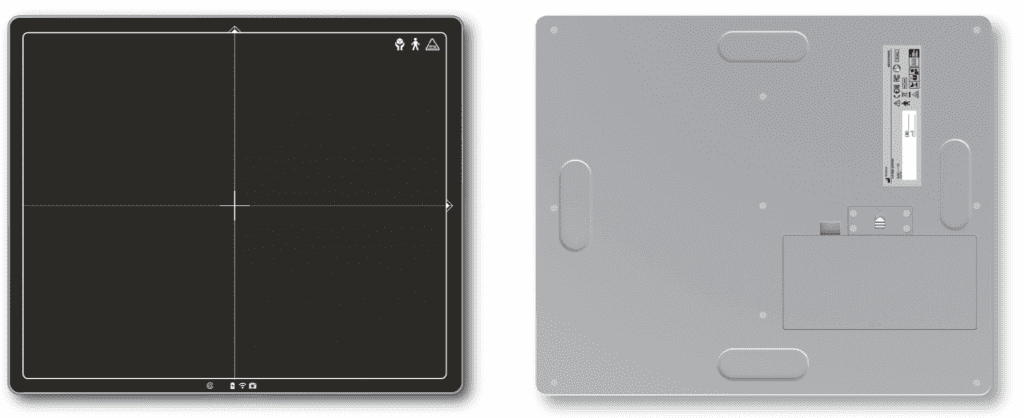
KA Imaging’s Flat Panel Sight™ has received a Canadian Medical Device Licence from Health Canada. With the license, the X-ray detector can be officially sold in Canada.
Among its main features, Sight™ can be called featherweight, as it weighs only 2.7 kilos in its 38 X 46cm version and 3.2 kilos in its 46 X 46cm version. In addition, Sight has a Wi-Fi option with a two-second image transfer. It is also IPX6 rated for water. AED allows use with any system.
“Sight™ is a powerful and affordable solution in the medical and veterinary fields”, says Karim S Karim, CTO of KA Imaging. “It is a product that came to complement KA’s line of solutions, so we can better address the different needs of radiologists, considering both technological and financial aspects,” says Amol Karnick, President and CEO of KA Imaging.