Kerecis® today announced MariGen® Shield, which integrates the company’s proven fish-skin graft with a silicone contact layer for treating chronic and complex wounds. The medical-fish-skin company also announced the results of a clinical study comparing the effectiveness of the Kerecis fish-skin grafts to a standard of care for diabetic foot ulcers. Both announcements were made at the Symposium on Advanced Wound Care (SAWC). Kerecis, which is pioneering the use of sustainably sourced fish skin and fatty acids in cellular therapy and tissue regeneration and protection, is exhibiting at booth 225.
New wound treatment combines fish skin graft and silicone
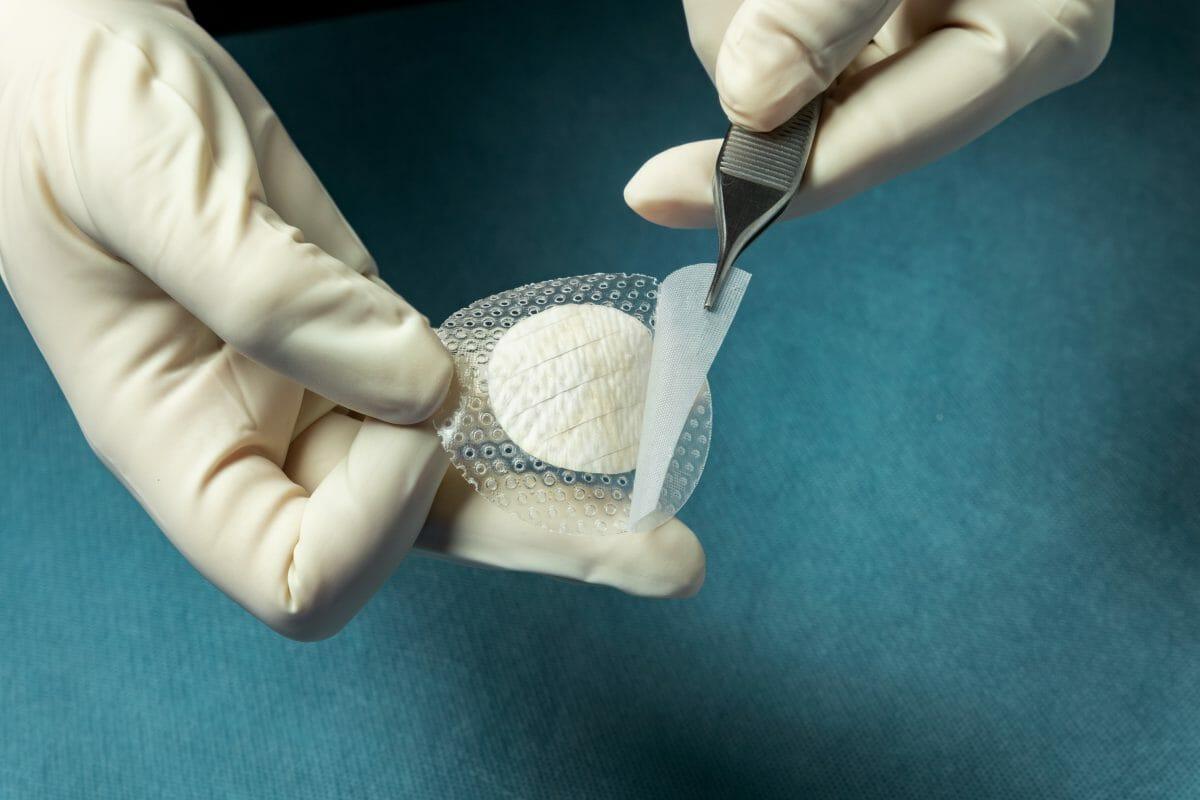
MariGen Shield integrates Kerecis’ medical-fish-skin graft with a silicone contact layer to provide an advanced treatment for chronic wounds. The product, which is fenestrated to allow adequate wound drainage, provides an optimal wound management environment and allows for sufficient wound moisture to facilitate healing.
The integrated, overlapping silicone contact layer holds the round graft in place and helps protect the granulating wound and the fish-skin graft after a cover dressing has been applied on top. This ease of handling speeds up application and removal. MariGen Shield is available in 20mm and 30mm round grafts, for easy application into foot wounds.
“This is the first time we have combined our fish skin with another material, in this case medical-grade silicone,” said Fertram Sigurjonsson, founder and CEO of Kerecis. “Until now the application of the Kerecis fish-skin graft has required three components: the fish skin itself, a contact layer and a cover dressing to regulate the moisture level on the wound. By integrating the contact layer with the fish skin, the application becomes faster and easier and the inventory needs at the healtcare facility simpler,” he added.
Comparative clinical study
Kerecis also announced the results of its prospective, randomized, single-masked, controlled clinical study comparing Kerecis fish-skin grafts with Fibracol™, a collagen alginate therapy. The multicenter, comparative study found that the MariGen healed almost twice as many non-responsive, chronic diabetic foot ulcers than the Fibracol therapy.
The researchers determined that treatment with the Kerecis fish-skin grafts should be considered “a more efficient and cost-effective solution” for treating diabetic foot ulcers than the alternative treatment.
The clinical study of 102 patients took place between June 2019 and January 2022 and involved 16 centers in the United States. The results are detailed in the paper “Final Efficacy and Cost Analysis of a Fish Skin Graft vs Standard of Care in the Management of Chronic Diabetic Foot Ulcers: A Prospective, Multicenter, Randomized Controlled Clinical Trial,” which was published in the April 2023 issue of the peer-reviewed journal Wounds. Other clinical studies have also determined that the Kerecis products heal wounds faster than alternative products.