Know Labs, Inc. (NYSE American: KNW), an emerging developer of non-invasive medical diagnostic technology, today announced the results of its proof-of-principle study titled, “Detecting Unique Analyte-Specific Radio Frequency Spectral Responses in Liquid Solutions – Implications for Non-Invasive Physiologic Monitoring,” conducted in partnership with a prestigious research institution out of Rochester, MN. Know Labs will present the results of the study at the American Physiological Society (APS) Summit, which is being held on April 20 – 23 in Long Beach, California. The study demonstrates the accuracy of Know Labs’ proprietary Bio-RFID™ sensor in quantifying different analytes in vitro, proving a 100% accuracy rate in these tests. The full study is currently undergoing the peer-review publishing process.
Know Labs’ electromagnetic technology platform, Bio-RFID, uses radio waves to non-invasively and safely penetrate surfaces, capture data from individual radio frequencies, and convert those data into physiologically meaningful information and insights. While the technology is proven to accurately measure several analytes inside and outside the body, the first application of this technology is aimed at non-invasive glucose monitoring.
“Proof-of-principle studies are critical in demonstrating Bio-RFID accuracy for non-invasive methods of medical diagnostics. This was an essential step toward achieving our goal of delivering the first FDA-cleared, truly non-invasive glucose monitoring device to the market,” said Ron Erickson, CEO and Chairman at Know Labs. “To put this into real-world context: imagine being able to continuously and accurately measure different aspects of your body on a molecular level using a pocket-sized (or smaller) sensor instead of a finger prick or a CGM probe.”
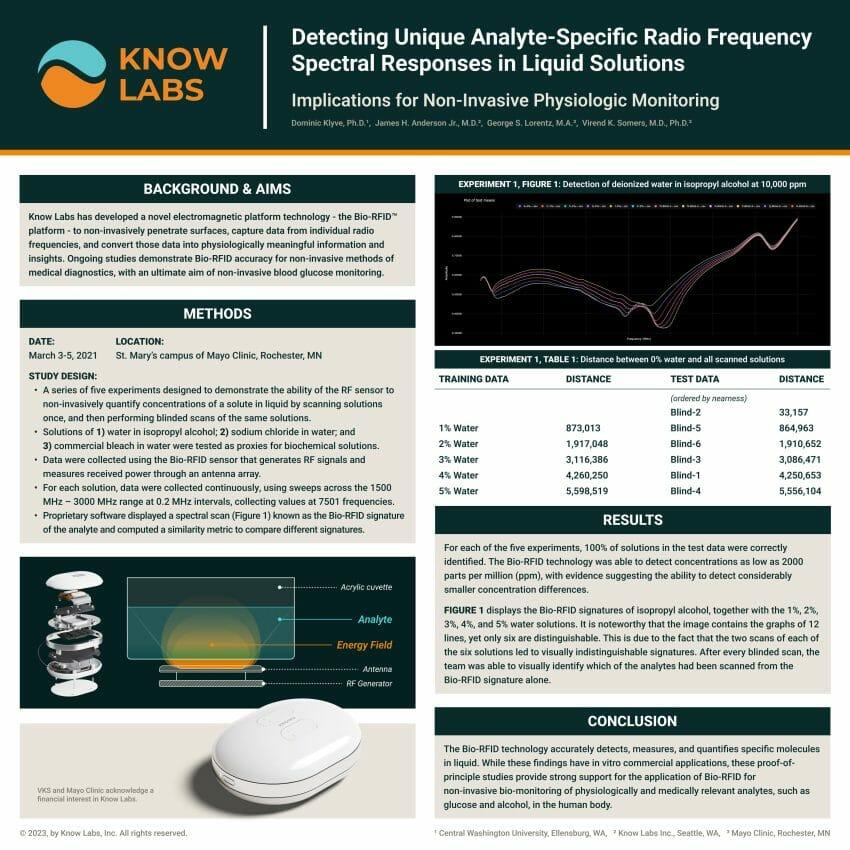
Conducted at the research institution’s Rochester, MN campus in March of 2021, the study included five experiments designed to demonstrate the ability of the RF sensor to non-invasively quantify concentrations of solutions using a randomized double-blind trial design, as proxies for biochemical solutions. Solutions including water in isopropyl alcohol, sodium chloride in water, and commercial bleach in water were tested. Data were collected using the Bio-RFID sensor, which generates RF signals and measures received power through an antenna array, using sweeps across the 1500 Megahertz (MHz) – 3000 MHz range at 0.2 MHz intervals, and collecting values at 7501 frequencies.
For each of the five experiments, 100% of solutions in the test data were correctly identified. The Bio-RFID technology was able to detect concentrations as low as 2000 parts per million (ppm) – which is equivalent to accurately measuring the difference of 0.7ml of water dropped into a 12oz soda can – with evidence suggesting the ability to detect much smaller concentration differences. These in vitro findings provide proof-of-principle that strongly supports the application of the technology for further non-invasive, physiologic and medical monitoring.
This proof-of-principle study is part of a series of studies that Know Labs is conducting as the company prioritizes external validation of its Bio-RFID technology in detecting and measuring glucose and other analytes in the body non-invasively at high levels of accuracy.
The study abstract and APS Summit Poster can be viewed at www.knowlabs.co. The full manuscript will be published following peer review.