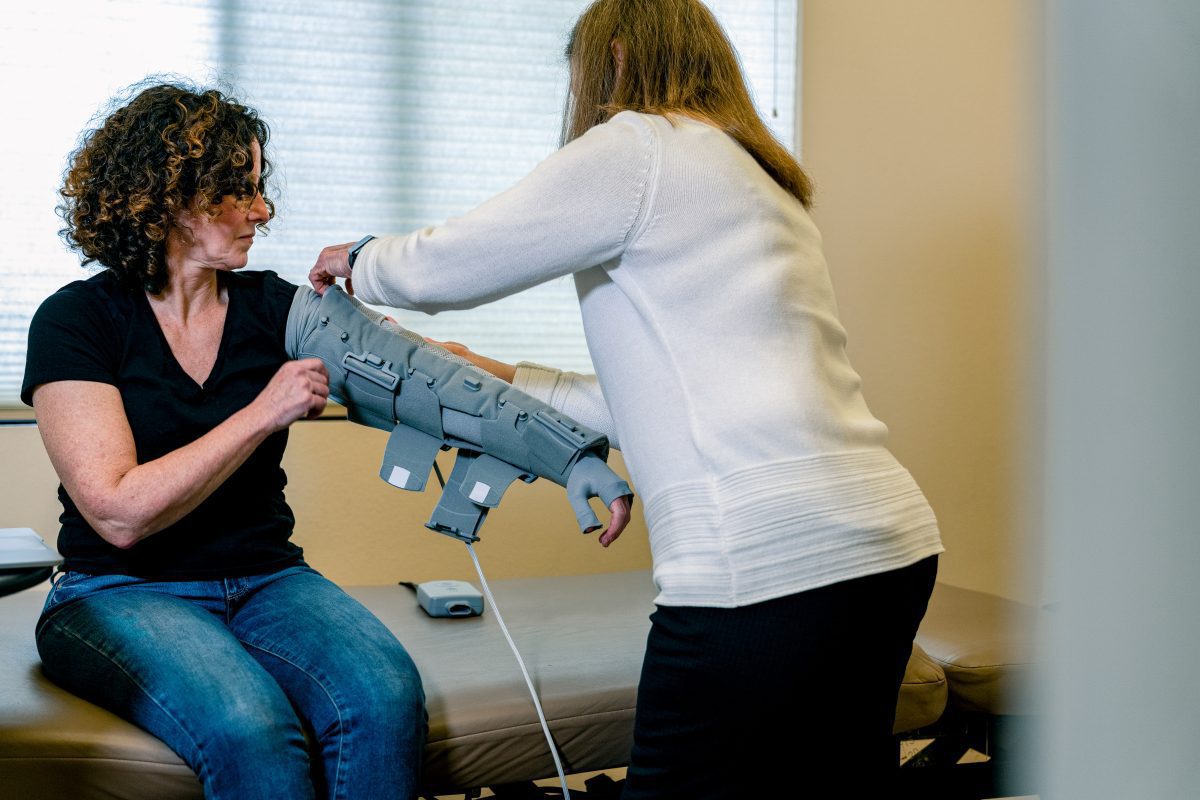
Koya Medical a healthcare company on a mission to transform lymphatic and venous care through a comprehensive suite of innovative, people-centric platforms, recently presented data from the company’s prospective, multi-center, randomized, crossover study (NILE – NCT04908254) comparing Dayspring®, the company’s active compression treatment for lymphedema, to a traditional pneumatic compression pump. In the trial, study participants reported significantly greater adherence to Dayspring treatment in comparison to the use of pneumatic compression pump. Additionally, subjects in the Dayspring arm demonstrated greater reduction in limb volume, improvement in quality of life and patient preference. Dayspring is the first active compression treatment that enables movement and mobility cleared by the U.S. Food & Drug Administration (FDA) to treat lymphedema and other similar conditions.
The data was presented late in the day on Friday, October 8, 2021 at the American Vein and Lymphatic Society (AVLS) 35th Annual Congress 2021 by Stanley G. Rockson, MD, Professor of Cardiovascular Medicine, Chief of Consultative Cardiology, and the Director of the Center for Lymphatic and Venous Disorders at Stanford University. Dr. Rockson also serves as chief medical officer of Koya Medical.
“For people with lymphedema, movement is important from both a clinical perspective and a quality-of-life perspective. These data illustrate the Dayspring treatment’s ability to provide that essential movement while still delivering the clinical outcomes we need from compression therapy,” said Dr. Rockson. “Traditional pneumatic compression pumps have long represented an important and effective tool for lymphedema patients to self-manage a lifelong chronic condition. It is rewarding to see this much-needed innovation coming to market; it provides patients with an additional option, especially this one that enables movement and improves adherence.”
The head-to-head trial has enrolled 52 participants thus far who were treated in the home sequentially, in random order, with either a Dayspring device or a leading pneumatic compression pump. Study participants were instructed to use the assigned randomized device at home for four weeks, one hour every day, and after a four week “wash-out” period were instructed to use the comparator device at home for four weeks, one hour every day. The interim data presented at AVLS represents 37 participants who have completed both arms of the study. Limb volume, quality-of-life outcomes, adherence, and patient preference were evaluated. Quality-of-life outcomes were assessed using the Lymphedema Quality-of-Life Questionnaire (LYMQOL), a validated disease-specific instrument to measure the impact of lymphedema on patients’ lives.
The primary endpoint of the study is non-inferiority for limb volume reduction against the comparator pneumatic compression pump. The interim analysis supported greater, statistically significant improvement in limb volume reduction (p<0.05). The study thus far has demonstrated statistically significant higher scores in the quality-of-life measure (p<0.05) for Dayspring when compared to the pneumatic compression pump. Also, the data showed stronger treatment adherence for Dayspring, with 95% of the study participants adhering to the prescribed treatment course, compared to only 48% of participants in the comparator group (p<0.01). Lastly, the majority of subjects (89%) preferred Dayspring over the pneumatic compression pump (p<0.01), reportedly due to movement, mobility and improved function in their daily lives.
These data contribute to the growing clinical support for Dayspring as a source of meaningful innovation in the treatment of lymphedema by enabling the patient to gain valuable movement and mobility during the treatment sessions – movement that is important to both quality of life and clinical outcomes.