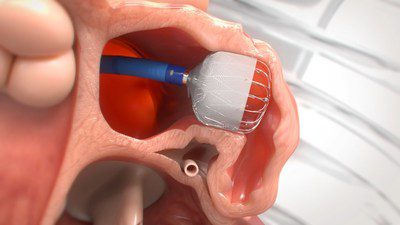
WATCHMAN FLX™ Left Atrial Appendage Closure Device Update
Today, Boston Scientific (NYSE: BSX) announced positive 24-month results from the PINNACLE FLX clinical trial assessing the safety and efficacy of the next-generation WATCHMAN FLX™ Left Atrial Appendage Closure (LAAC) Device for patients with non-valvular atrial fibrillation (NVAF). Presented as late-breaking clinical science at TVT: The Structural Heart Summit, the study evaluated the WATCHMAN FLX device as an alternative to long-term oral anticoagulation therapy, including non-vitamin K antagonist oral anticoagulants (NOACs), for stroke risk reduction in patients with NVAF.
The prospective, non-randomized PINNACLE FLX trial included 400 patients in the U.S. with NVAF who were eligible for anti-coagulation therapy to reduce the risk of stroke but had appropriate rationale to seek a non-pharmaceutical alternative. Following the positive 12-month results in which the trial met its primary safety and efficacy endpoints, the trial met its secondary effectiveness endpoint – defined as the occurrence of ischemic stroke or systemic embolism over 24 months – with a rate of 3.4% compared to the performance goal of 8.7%.[i]
“These findings demonstrate sustained device performance over two years and reinforce the excellent safety and efficacy profile of the WATCHMAN FLX technology,” said Saibal Kar, M.D., study co-principal investigator and interventional cardiologist at Los Robles Regional Medical Center and Bakersfield Heart Hospital, California. “Building upon the low complication rates and 100% rate of effective LAA closure seen at 12 months, the 3.4% rate of ischemic stroke and systemic embolism at 24 months is very encouraging in this complex, elderly patient population.”
In addition to the low rate of ischemic stroke, the data through 24 months also demonstrated that no patients experienced a device embolization or pericardial effusion requiring cardiac surgery, all of which is favorable in the context of previous clinical studies.[ii]
“The final results of this pivotal study underscore how design advancements of the WATCHMAN FLX device – which allow for improved anchoring, a faster, more effective LAA closure and compatibility with more complex anatomies – have translated into a safe, effective and durable option for patients with NVAF at increased risk for stroke and systemic embolism and an appropriate rationale to seek a non-pharmaceutical alternative,” said Dr. Ian Meredith, AM, global chief medical officer, Boston Scientific.
The next-generation WATCHMAN FLX device received U.S. Food and Drug Administration (FDA) approval in July 2020 and CE Mark in March 2019, and is now used in nearly all implants in the U.S. and Europe in lieu of the previous-generation device.
The company continues its clinical research on the WATCHMAN FLX device for use in patients with NVAF via two large prospective, randomized controlled trials: the OPTION trial – comparing the WATCHMAN FLX device to oral anticoagulants in patients who also undergo a cardiac ablation procedure; and the CHAMPION-AF clinical trial – studying a broader anticoagulant-eligible patient population to evaluate the device against NOACs for embolic stroke prevention.
For more information on the WATCHMAN FLX device, visit www.watchman.com/implanter.