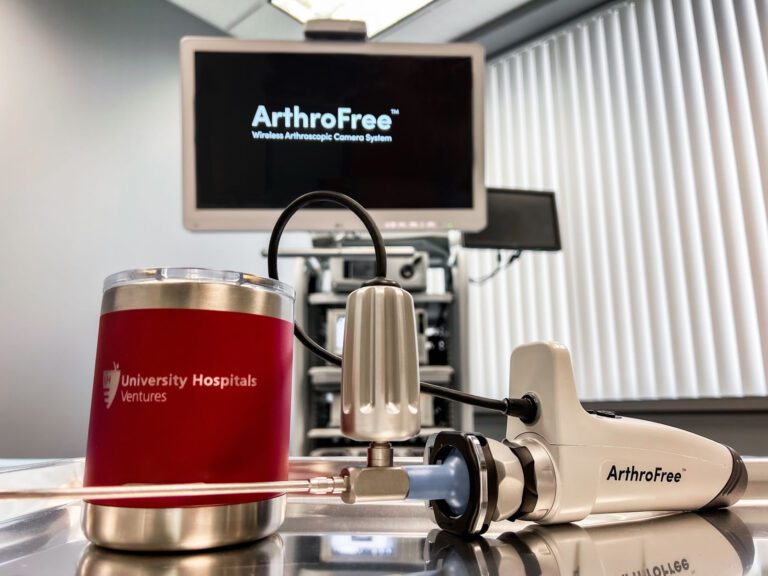
Lazurite Holdings LLC today announced a new phase of collaboration with University Hospitals (UH) Ventures, the innovation and commercialization arm of University Hospitals Health System in Cleveland, which will include an investment from UH Ventures. The amount and details of the investment were not specified.
This past August, Lazurite Holdings announced its collaboration with UH Ventures on a human factors study of the ArthroFree™ system and said it hoped the study would be the beginning of a broader, longer-term collaboration.
“This partnership is a great example of the way in which we can work to progress technologies from our local region. We look forward to exploring clinical studies with the Lazurite team and continuing to expand the relationship,” said David Sylvan, President of UH Ventures.
The ArthroFree™ system is expected to be the first wireless surgical camera system for the minimally invasive operating room approved by the U.S. Food and Drug Administration (FDA). The system incorporates the company’s proprietary low-heat, high-intensity Meridiem™ light technology along with advanced camera, battery, and wireless transmission technologies. The system is designed to deliver improved operating room productivity, patient safety, and economic value through cost-savings, energy efficiency, and reduced setup/breakdown times. The modular system also is designed to be fully drop-in compatible with current operating room technology. Approval by the FDA is expected by mid-year 2022.
“We are excited to have UH Ventures as an investor and to work with UH’s surgeons and surgical staff as our partners in additional studies and research related to the ArthroFree™ system and future products we are developing,” said Lazurite Holdings President Leah Brownlee. “I believe this collaboration will be critical to our success and will further advance University Hospitals’ reputation as an innovative leader in advancing new medical technology.”
The ArthroFree™ system will be available for viewing and hands-on demonstration at three upcoming conferences:
The Annual Meeting of the Society of American Gastrointestinal and Endoscopic Surgeons (SAGES), March 16-19, in Denver (Booth 202)
The Annual Meeting of the American Academy of Orthopaedic Surgeons (AAOS), March 22-26, in Chicago (Lazurite Booth 2305)
The Annual Conference & Expo of the Association of periOperative Registered Nurses (AORN), New Orleans, March 19-23 (Booth 7252)
The ArthroFree™ wireless surgical camera system has not yet received FDA clearance and is not currently approved for human use. It is not intended for commercial distribution; orders cannot be accepted at this time.