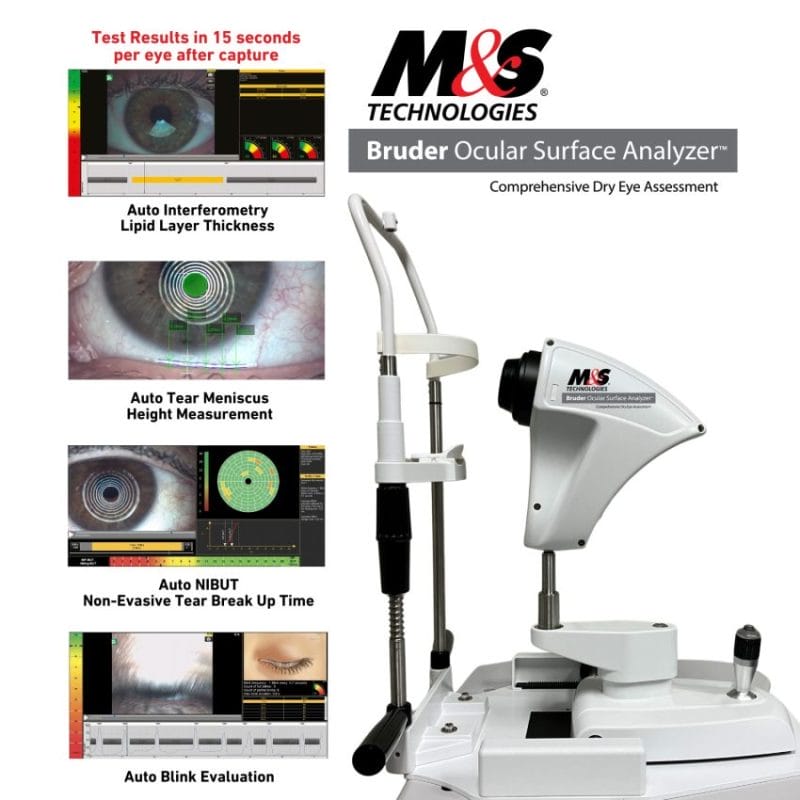
M&S Bruder Ocular Surface Analyzer (BOSA)
M&S Technologies, a leader in computerized vision testing in collaboration with Bruder Healthcare, renowned for dry eye solutions announces the launch of the M&S Bruder Ocular Surface Analyzer (BOSA). The all-in-one innovative device represents an advancement in dry eye assessment and is poised to be a necessity for eye care practices treating ocular surface disease (OSD), including dry eye syndrome. In only 15 seconds per eye, BOSA captures and provides OSD test results. “With the system’s unmatched speed, accuracy, and ease of use, doctors can manage dry eye patients more efficiently and effectively, improving outcomes through better data and boosting revenues through time savings,” explains Brent Jones of Hilco Vision, parent company of M&S Technologies and Bruder Healthcare.
Clinician Benefits
Based on the patient’s level of disease, BOSA offers clinicians the ability to create customizable treatment plans which can include their existing dry eye treatment technology, prescribed therapeutics, and at-home therapy. “BOSA provides a ready-made hub to help identify, quantify and rectify OSD – with recommended treatments tailored to each patient’s test results,” said Paul Karpecki, OD, FAAO. Since BOSA saves test results of each session, clinicians can compare test results over time, illustrating patient progress while motivating them to continue treatment.
With over 50 million individuals in the United States experiencing dry eye symptoms, and 60 percent of eye care professionals seeking to expand their OSD services, BOSA arrives as a game-changer, transforming the ways ophthalmologists and optometrists measure dry eye by providing precision and accuracy to the equation. Traditionally, assessing dry eye involved multiple tests, in some cases not feasible within a single practice location. BOSA consolidates these diverse tests into one efficient device, offering eye doctors a comprehensive solution within their exam rooms. BOSA’s array of non-invasive tests includes four tests with results generated in 15 seconds per eye after capture: Auto Interferometry for Lipid Layer Thickness, Auto Tear Meniscus Height, Auto Tear Breakup Time (TBUT) and Auto Blink Evaluation. BOSA performs six other tests in minutes, including Auto Meibography (Gland Detection), Bulbar Redness, Fluorescein Staining, Lissamine Green Staining, Pupillometry test, and White to White test. BOSA also provides documentation for Demodex and Blepharitis.
BOSA also offers a clear, understandable patient report, reinforcing treatment recommendations and aiding in patient education. “The Bruder Ocular Surface Analyzer is the most comprehensive “one-stop” instrument available to document Dry Eye Disease. It is the answer for both the established Dry Eye practice looking to improve efficiency and the eye care practice looking to start a Dry Eye clinic,” said Darrell White, MD of SkyVision Centers.
M&S Technologies and Bruder Healthcare account representatives have begun meeting with doctors to provide information about BOSA tailored to their practices including customized treatment plans, cost/benefit analyses, and financing options, including the option for doctors to purchase BOSA with Bruder Healthcare product bundles, synching up with their preferred treatment protocols, for additional savings and efficiency.
Delivery
M&S BOSA is set for delivery in early 2024. Interested eye care professionals can contact their M&S or Bruder Healthcare account representatives.