LFM Healthcare Solutions, LLC, has released its full report of U.S. Food & Drug Administration “MAUDE” data revealing that the number of medical device reports involving “patient-ready” flexible endoscopes that could have potentially exposed patients to infectious pathogens has risen sharply over the past eight years and continued to rise in 2021.
The study also notes that these “adverse-event reports” filed by hospitals, endoscope manufacturers, patients and other stakeholders rose markedly in all categories of studied endoscopes from 2014 to 2021 and continued to “increase significantly” from 2019 to 2020 for urological endoscopes and colonoscopes “when the number of elective or non-urgent endoscopic procedures performed during the pandemic decrease in the U.S. and globally ….”
“While endoscope procedures are generally quite safe, my research found a concerning trend, that patients continue to face the threat of being exposed to a superbug from an endoscope if it isn’t properly cleaned,” said Lawrence F Muscarella, PhD, President of LFM Healthcare Solutions, LLC, a Lansdale, PA-based independent infection prevention and quality improvement company. “Ineffective cleaning of flexible endoscopes is a public health worry that can significantly increase a patient’s risk of acquiring a healthcare-associated infection.”
“Superbugs” are organisms that are resistant to multiple types of antibiotics. Some superbugs today have developed resistance even to “last-resort” antibiotics, such as carbapenems.
Dr. Muscarella spent more than six months manually reviewing more than 10,000 adverse-event reports submitted to the FDA’s Manufacturer and User Facility Device Experience database, or MAUDE. Reports are submitted by manufacturers and healthcare facilities in accordance with mandatory federal requirements, and by voluntary reporters, including patients and consumers.
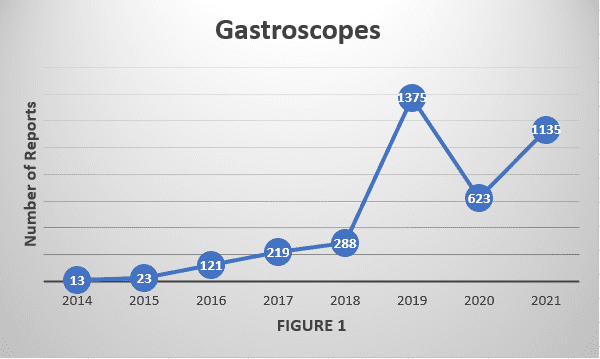
His detailed investigation, Contamination of Flexible Endoscopes and Associated Infections: A Comprehensive Review of Adverse-Event Reports Submitted to FDA, found that for all six of the studied endoscope types, the number of counted reports satisfying this analysis’s inclusion criteria – those FDA reports describing actual or potential contamination of a reprocessed endoscope — has increased significantly since 2014.
The increase was most marked for gastroscopes1,2; however, whose number of relevant reports rose more than 80 percent from 2020 to 2021 alone. The significant rise in adverse-event reports for gastroscopes, and similarly for colonoscopes too during this same timeframe, have not been directly addressed in a recent FDA safety communication, Dr. Muscarella said.
“Such an educational notice with specific safety recommendations would help to reduce the infection risk and bring attention to specific missteps that sometimes can be overlooked when resources are limited – for example, inadvertently failing to reprocess every potentially contaminated endoscope channel,” Dr. Muscarella added.