LG: Spurred by the pandemic and the larger trend toward remote work, radiologists have started seeking display solutions that allow them to read critical medical images from anywhere.
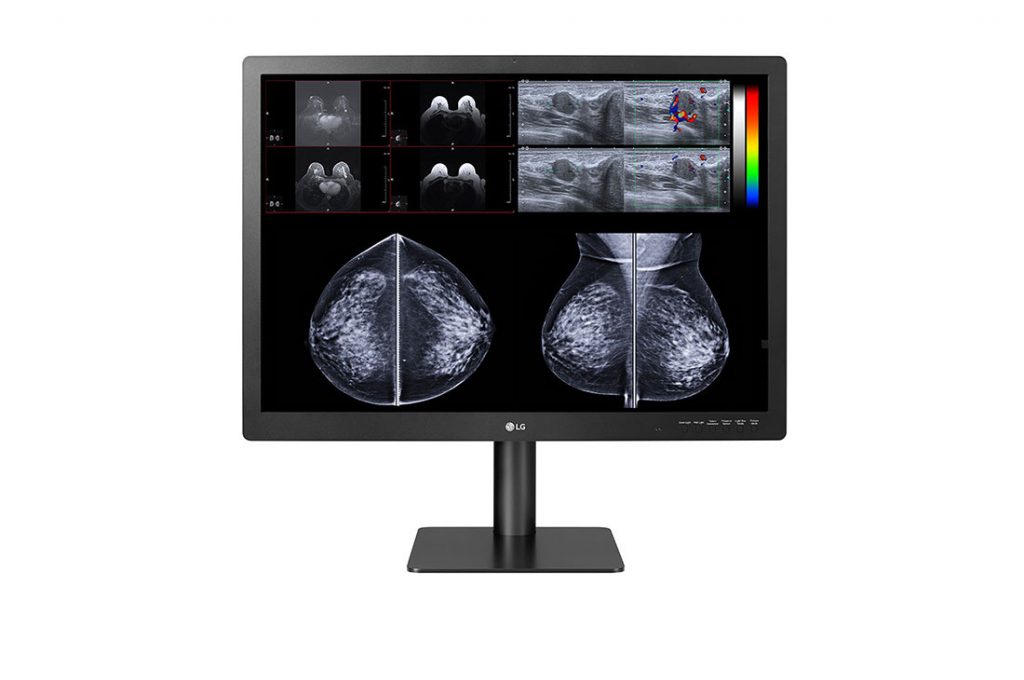
To support their work, LG Business Solutions USA has announced the latest in LG’s full line of professional, medical-grade diagnostic monitors, the 31-inch, 12-megapixel 31HN713D, which can be used for multiple modes of diagnostic study, including 3D mammography.
“More and more, radiologists and tele-radiologists working remotely have embraced the high performance and cost-effectiveness of LG medical monitors to do their jobs more efficiently,” said Stephen K. Hu, head of medical monitors at LG Business Solutions USA. “With this new model, they have a diagnostic monitor capable of even the most challenging studies, plus features to help maximize their productivity. Its multimodality capabilities allow physicians to read studies from all modalities (Mammography, CR, DR, CT, MR, Ultrasound, etc.) making it a perfect choice for use in healthcare facilities and home offices alike.”
Traditionally, mammography has represented a challenge to digital imaging and diagnostics, requiring high brightness and resolution to discern subtle details in the human breast. Hu said the new model is optimized for breast imaging, with an ultrafine 4200×2800 IPS panel capable of up to 1080cd/m2 of brightness. In addition, its 31-inch screen size and 12MP resolution allow the monitor to take the place of two 5MP medical monitors commonly used for diagnostic mammography. LG’s IPS technology supports wide viewing angles, helping to ensure more accurate viewing of multiple images laid out on the same monitor.
“With LG’s ability to support diagnostic mammography along with all other readings, from X-rays, to CT scans, to ultrasounds, we’ve made it easy for radiologists to find a medical monitor to meet all radiologists’ needs,” Hu said. “This new monitor even includes a multi-resolution mode for switching between 6 and 12 megapixels depending on the imagery, plus a set of hot keys for toggling quickly among modes, resolutions, and light settings. This real-time multimodality supports a streamlined, efficient diagnostic workflow.”
The FDA 510(k)-certified and DICOM-compliant LG 31HN713D diagnostic monitor meets parameters set by the American College of Radiology for image quality in digital mammography. It comes with an integrated front sensor for automated self-calibration to ensure accurate, consistent imagery without requiring additional calibration equipment. It also has a built-in presence sensor that turns off the display when not being used, as well as an auto-luminance sensor to optimize screen brightness for ambient lighting conditions.
Helping radiologists perform better readings, the new monitor features a pair of special diagnostic modes. The Focus View mode allows radiologists to review sections of a medical image by adjusting brightness and grayscale tones in the area of focus while darkening the rest of the screen. The Pathology mode emulates the level of detail and color viewable under a microscope for diagnoses.
The LG 31HN713D also includes built-in down and wall lighting to help improve contrast and better illuminate radiologists’ work surfaces when viewing images in dark spaces.