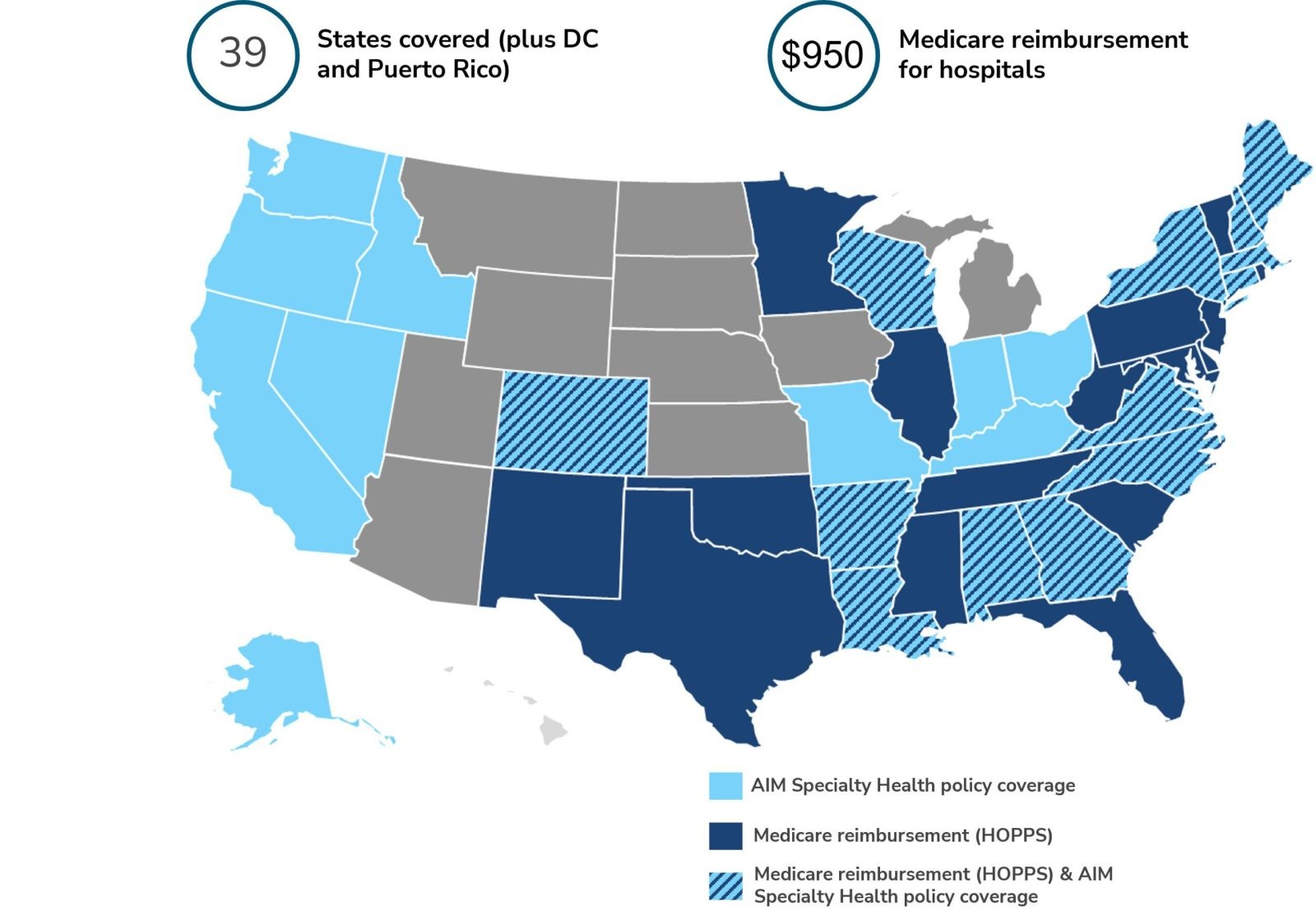
LiverMultiScan: AIM Specialty Health deems LiverMultiScan medically necessary for evaluating diffuse liver diseases, covering over 77 million US lives. This new AIM Advanced Imaging guideline includes the stand-alone CPT Code 0648T used when billing for the LiverMultiScan procedure.
“These advances in coverage and reimbursement for LiverMultiScan procedures have the potential to help change the paradigm of liver disease care by providing broader access to patients eligible in California,” Mazen Noureddin, MD, MHSc, Cedars-Sinai Medical Center, Los Angeles, California.
LiverMultiScan is a non-invasive MRI technology that delivers a clear picture of liver health in one single scan through three key metrics – fibro-inflammation, fat, and iron – and may be an alternative to liver biopsy.1 Studies have demonstrated that LiverMultiScan is a non-invasive, clinically accurate diagnostic for identifying patients with disease activity at the point where the disease is still reversable.2 With broad coverage and reimbursement options available, more than ever before, clinicians and patients can conquer liver disease through rapid and reliable imaging with LiverMultiScan.
The new guidance directs Radiology Benefit Management decisions within the following health plans: Anthem (California, New York, Colorado, Connecticut, Georgia, Indiana, Kentucky, Missouri, Ohio, Wisconsin, Maine, New Hampshire, Nevada, Virginia), BlueCross BlueShield Alabama, BlueCross BlueShield Arkansas, BlueCross BlueShield Louisiana, BlueCross BlueShield Massachusetts, BlueCross BlueShield North Carolina, LifeWise Health Plans, Premera, and Pacificsource.
In addition, the following Medicare Administrative Contractors (MACs) have updated Hospital Outpatient fee schedules to include reimbursement of LiverMultiScan when ordered and billed as a stand-alone procedure under CPT Code 0648T: Novitas Solutions, National Government Services, Palmetto GBA, and First Coast Service Options.
Additionally, the Perspectum Coverage Support (PCS) Program assists and increases patient access to the LiverMultiScan through submission for prior authorization and patient-based appeals.
Access the New AIM Advanced Imaging guideline.
References
1Andrea Dennis et al., “Correlations Between MRI Biomarkers PDFF and CT1 With Histopathological Features of Non-Alcoholic Steatohepatitis,” Frontiers in Endocrinology 11 (2021): 1053, https://doi.org/10.3389/fendo.2020.575843.
2Anneli Andersson et al., “Clinical Utility of MRI Biomarkers for Identifying NASH Patients’ High Risk of Progression: A Multi-Center Pooled Data and Meta-Analysis,” Clinical Gastroenterology and Hepatology: The Official Clinical Practice Journal of the American Gastroenterological Association, October 6, 2021, S1542-3565(21)01056-9, https://doi.org/10.1016/j.cgh.2021.09.041.