Lunit (KRX:328130.KQ), a leading provider of AI-powered solutions for cancer diagnostics and therapeutics, announces two commercial deals that signal its expansion in Europe. Lunit has secured a supply contract with TeleDiag in France and the Portuguese League Against Cancer (Liga Portuguesa Contra o Cancro; LPCC), providing AI-powered radiology solutions for chest X-ray and breast cancer detection, respectively.
TeleDiag and Lunit Partner for Accurate Lung Abnormalities Detection in France
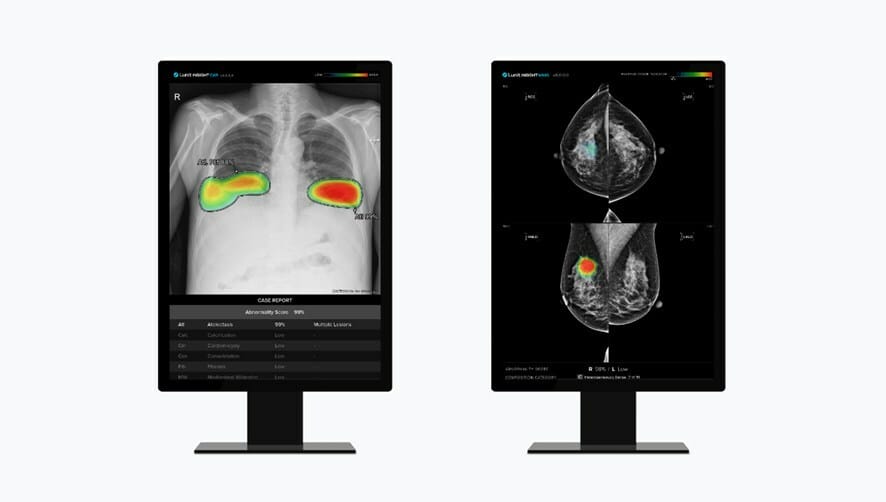
unit has entered into a supply contract with TeleDiag, France’s largest teleradiology group, established in 2008. By delivering Lunit INSIGHT CXR, a CE-marked AI-powered solution to detect 10 of the most common lung abnormalities, including lung cancer, Lunit and TeleDiag aim to enhance the accuracy and effectiveness of lung disease detection. As TeleDiag regroups a vast network of over 600 radiologists serving more than 300 medical practices and screening over 600,000 patients annually, the collaboration represents a significant leap forward in the integration of AI technology into the French healthcare system.
The decision to choose Lunit came after TeleDiag conducted a thorough evaluation of multiple AI solutions for chest X-rays. Lunit emerged as the top choice due to its robust technical performance and its wider range of findings.
LPCC Partners with Lunit for AI-driven Mammography Analysis in Portugal
In parallel, Lunit has inked a supply agreement with the central region branch of the Portuguese League Against Cancer (LPCC), to deliver its FDA-cleared and CE-marked AI-powered solution for mammography analysis, Lunit INSIGHT MMG. The central region branch of LPCC plans to analyze about 100,000 mammograms annually for the next three years using Lunit INSIGHT MMG.
Founded in 1941, LPCC is a private, non-profit, non-governmental organization overseeing the Portuguese National Breast Cancer Screening Program. LPCC decided to purchase Lunit’s solutions after an extensive internal validation process using their own population screening data, where Lunit demonstrated exceptionally high performance.
“Last week, we announced new supply contracts in East and Southeast Asia. This week, we unveil our new collaboration with TeleDiag and the Portuguese League Against Cancer in Europe; we’re not just expanding our reach; we’re transforming diagnostics globally,” said Brandon Suh, CEO of Lunit. “Our aim is clear: to bring our AI-powered cancer screening solutions directly to healthcare providers, improving patient outcomes, ensuring that the benefits reach every corner of the world.”