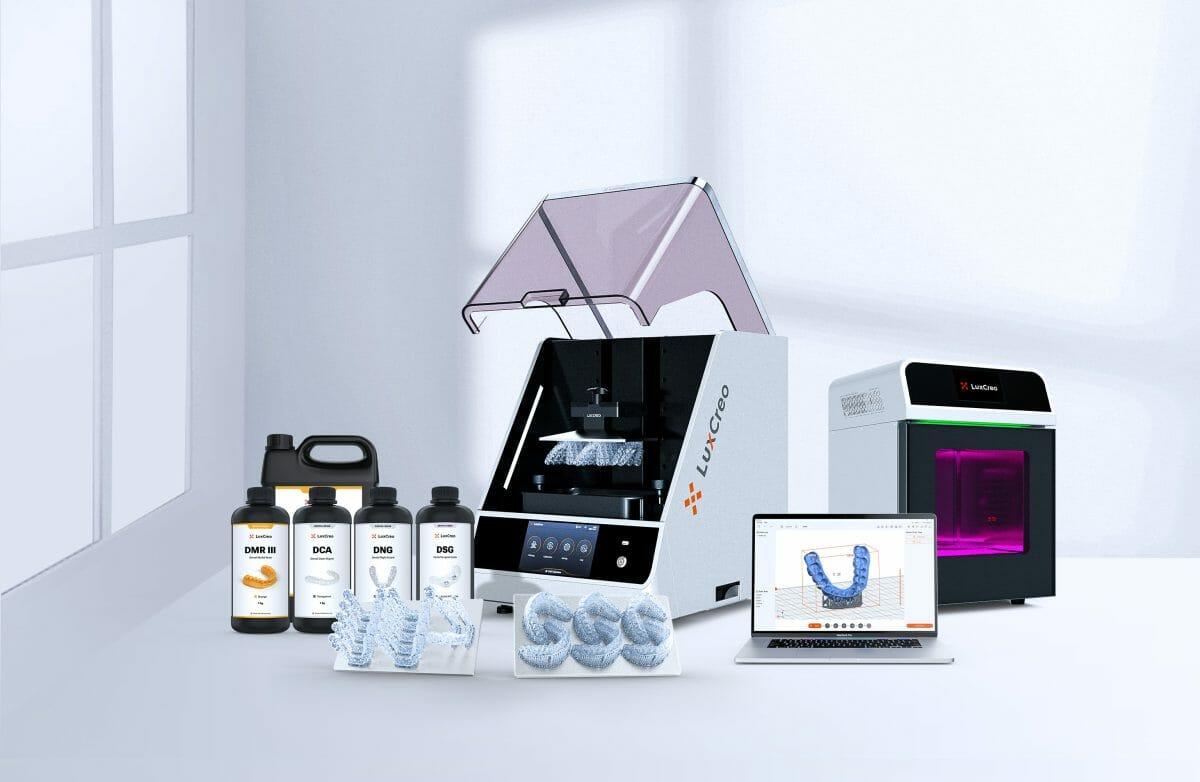
LuxCreo, a leading 3D printing specialist in clear dental appliances, announced today an FDA Class II 510(k) cleared end-to-end solution for orthodontists and dentists to produce same-day clear aligners, bypassing traditional model and thermoforming stages. This approach enables orthodontists and dentists to deliver aligners to patients in as little as 2 hours from an oral scan with significant labor and CapEx savings compared to in-house thermoforming.
LuxCreo CEO Mike Yang said:
“After 5 years of collaboration with orthodontists, dentists, and dental labs, we are excited to introduce our integrated suite of direct aligner digital fabrication tools. Direct print aligners offer higher accuracy compared to thermoformed alternatives which can result in a better patient fit. Furthermore, our patent-pending multi-thickness technology enables customized teeth movements tailored to each patient’s unique treatment needs.”
In Jan 2023, Fortune Business Insights forecasted that the global clear aligner market to skyrocket from $4.1 billion in 2022 to $16.1 billion in 2029. Despite this growth, LuxCreo estimates that 5-10% of aligner treatments are canceled by patients due to lengthy initial aligner lead times—up to 6 weeks. LuxAlign’s platform addresses these issues, enabling patients to try on their first aligners as fast as 2 hours after intraoral scanning at the dentist’s office.
To deliver same-day direct clear aligners capabilities, LuxCreo is launching a suite of products across hardware, software, materials, and services to enable a seamless end-to-end workflow which includes:
- iLux Pro Dental: A high throughput, small footprint, heated, chairside DLP printer with 50-micron pixel resolution powered by LEAP™ technology. A single iLux Pro Dental is capable of printing up to 175 models horizontally or up to 64 direct aligners in an 8-hour shift.
- LuxAlign® design software: Automatically generate aligner retainer designs, add trimlines, repair tooth gaps and vary aligner thickness for enhancing aligner efficacy and patient comfort.
- iLuxCure Pro: A programmable, multi-wavelength, multi-direction, high-irradiance post-print cure that can cure 6-8 aligners within 30 minutes.
- Direct Clear Aligner Material (DCA): DCA is a tough, flexible, and accurate clear aligner material with high transparency without manual polishing, enabled by LuxCreo’s Digital Polishing™ technology. DCA has been FDA Class II 510(k) cleared for an aligner indication.
- LuxScale: After patients receive and approve their initial aligners, dentists can order or modify the remaining aligner treatment plan from a LuxCreo smart factory.
“The iLux Pro Dental and LuxAlign design software has been a game changer for my practice; I’ve eliminated the need to print models, thermoform them and cut the aligners. LuxAlign has saved my practice 60-75% in labor costs compared to the traditional workflow and has slashed waiting times for my patients.” Dr. Mike Pham, CEO of Affordable Dental.
The iLux Pro Dental 3D printer and iLuxCure Pro are available now for order and will begin shipping next month.
LuxCreo has also partnered with leading dental lab, Orthodent Laboratory (ODL) to clinically validate the efficacy of multi-thickness aligner technology and is actively seeking participants. For more information, visit the LuxCreo booth #695 at AAO in Chicago from April 21-24 or visit www.luxcreo.com