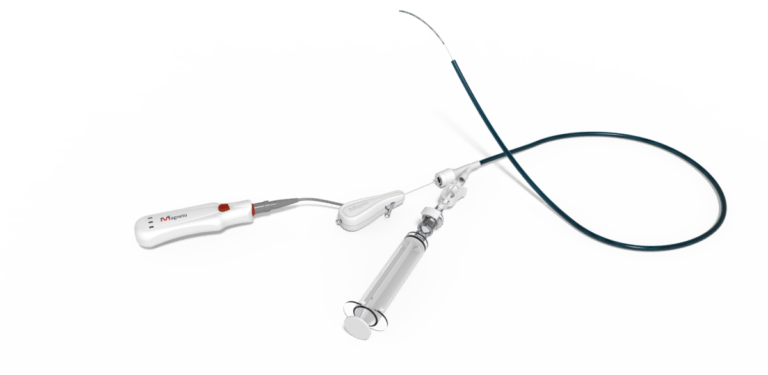
Magneto Thrombectomy Solutions (Magneto), a medical devices company developing innovative thrombectomy solutions for the treatment of ischemic stroke and pulmonary embolism, presented today successful first-in-human (FIH) results showing safety and feasibility of the eTrieve™ system, a novel catheter-based on electric fields for removing blood clots, in patients with acute pulmonary embolism (PE).
The data, presented today at Biomed Israel 2022, the premier international Life Science and HealthTech conference in Israel (May 10-12, Tel Aviv), showed a significant reduction in RV/LV ratio and alleviation of PE associated symptoms.
Magneto was established as part of Incentive, Peregrine Ventures’ Incubator, based on technology that was licensed from BGN Technologies, the Technology Transfer Company of Ben-Gurion University of the Negev (BGU). Peregrine Ventures has been supporting Magneto since its inception.
“We are very pleased with our FIH results, which confirm the safety and efficiency of our innovative technology, as previously demonstrated in preclinical studies,” said Benny Dilmoney, CEO of Magneto Thrombectomy Solutions. “Our novel catheter is a groundbreaking solution that can transform treatment of conditions caused by blood clots, such as pulmonary embolism. The technology generates strong attachment forces between the eTrieve™ catheter and the clot, allowing the effective removal of the clot without applying outward radial forces against the vessel wall, minimizing bleeding and enabling removal of both proximal and distal clots. We believe that our novel solution will be a game changer in removing clots and helping people recover safely from life-threatening conditions such as PE. We look forward to initiating our pivotal study on more than 100 patients in multiple medical centers in the US and Europe, planned for later this year.”
“Magneto provides intermediate and high-risk PE patients with a unique solution for safely and effectively removing large clots blocking the arteries in the lungs,” said Prof. Piotr Musialek, John Paul II Hospital, Krakow, Poland and an investigator in the study. “The combination of a large-diameter ‘vacuum cleaner’ with electro-mechanical thrombus extraction, allows an immediate resolution of dyspnea and swift recovery of the cardio-respiratory system.”
The prospective, single-arm, multicenter first-in-human study assessed the initial safety and performance of eTrieve™ in treating patients with acute PE. The study enrolled 10 participants across three sites in Denmark and Poland. Results show that the procedure, performed under light sedation only, was safe with no device related complications.
The primary performance endpoint was also met, with significant reduction of right ventricle to left ventricle volume 48 hours post intervention. Significant clot volumes were removed, and alleviation of PE associated symptoms was demonstrated. The catheter removed both fresh and organized clots and successfully removed blood clots of all sizes and types, from locations that could not have been safely reached using other techniques.
Prof. Asger Andersen, Aarhus University Hospital, Aarhus, Denmark, principal investigator of the study, added, “Large bore aspiration of central clots with possibility to retrieve segmental clots with the eTrieve™ catheter, optimizes the possibility for complete pulmonary revascularization in acute PE.”
About eTrieve™
The eTrieve™ is a state-of-the-art catheter for performing thrombectomy utilizing the electric properties of blood clots. When voltage is applied to the catheter, the negatively charged clot bonds to the positively charged electrode of the catheter, creating a strong grip, and allowing safe removal of the clot.
A pulmonary embolism (PE) is a blood clot that develops in a blood vessel in the body (often in the leg), travels to a lung artery and suddenly blocks blood flow. About 400,000 patients in the US are diagnosed with PE each year. Of these, approximately 200,000 patients have PE that is severe enough to cause right heart strain and can benefit from catheter-based treatment. PE with right heart strain is associated with a large clot burden, high acute mortality, and poor long-term prognosis. PE is a rapidly growing market enhanced by the Pulmonary Embolism Response Team (PERT) approach for PE management. The Potential US Market is estimated at $2 Billion.
Magneto Thrombectomy Solutions is developing innovative thrombectomy solutions for the treatment of ischemic strokes and pulmonary embolisms. The Company’s device enables fast clot retrieval with a small profile device having minimal interaction with the vessel wall while maintaining a strong grip throughout the length of the clot. The Company was established as part of Incentive, Peregrine Ventures’ Incubator, by Yuval Taff, Chief Technical Officer and Founder, based on technology developed by Prof. Itzhak Orion of BGU and licensed from BGN Technologies, the Technology Transfer Company of Ben-Gurion University of the Negev, Israel.