Mayo Clinic: The New Mayo Clinic Diet, the official diet developed by the Mayo Clinic, has launched nationwide. Through a medically-supported, scientifically rigorous and proven approach, The New Mayo Clinic Diet helps people lose weight to improve their health.
Built by a team of weight-loss experts at the Mayo Clinic, the program is built on a powerful new digital behavior change platform. The New Mayo Clinic Diet is designed to help members reshape their lifestyle by adopting healthy new habits and breaking unhealthy old ones.
The program includes Mayo Clinic-approved meal plans and recipes, based on a weight loss solution that consistently ranks on the US News & World Report Best Diet list and #1 on The New York Times bestseller list.
Members can choose from flexible meal plans, vegetarian, Mediterranean, high protein, and a new healthy keto program.
“The New Mayo Clinic Diet partners a whole-health weight loss program with an innovative digital platform.” said Donald D. Hensrud, MD, MS, Medical Director of the Mayo Clinic Healthy Living Program.”
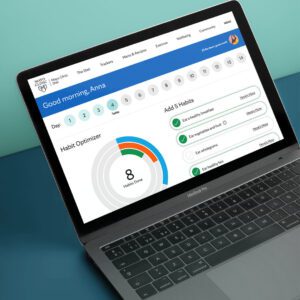
The New Mayo Clinic Diet is built on an all-new digital platform from Digital Wellness, which has tools and trackers to help members lose 3x more weight. Exclusive tools such as a Habit Optimizer help members make lasting meaningful changes to their behavior. The program also includes unlimited access to a private Facebook Group that helps members connect and support each other.
The New Mayo Clinic Diet features:
- Personalized Mayo Clinic-approved meal plans and recipes, based on the #1 New York Times best-selling diet that consistently ranks as a US News & World Report Best Diet
- Holistic and whole self-focused program – Calorie counting not required
- Food tracker with 1,000,000+ database
- Equipment-free workouts
- Tools to track progress and log your meals, exercise, measurements, and body weight
- Access to the unparalleled educational content and expertise of the Mayo Clinic.
- Mayo Clinic Diet 12-Week Course to help you learn the principles of healthy weight loss
“Another evolutionary element of the new Mayo Diet is a focus on the psychological aspects of weight management,” said Hensrud. “The new Mayo Clinic Diet Assessment assesses a participant’s diet mindset and gives them positive reassurance and mental support as they undertake a lifestyle change that, for many, can be very, very challenging.”