Mesuron announced today that it has entered into a know-how collaboration agreement and stock purchase agreement with Mayo Clinic to further refine its protocol algorithm and analytic software for the AVALON-H90’s Ventricle Repolarization Dynamics Analysis (VRDA) output, specifically for Acute Chest Pain (ACP) patients admitted to the hospitals’ Emergency Rooms (ERs) in order to rapidly separate ACP caused by heart problems from other causes not related to the heart. As part of the agreement, Mayo Clinic became a shareholder in Mesuron Inc.
Mesuron Inc. is a U.S.-based company specializing in the development and fabrication of Superconducting Quantum Interference Device (SQUID)-based magnetometers for various applications. The use of this technology for heart screening is the company’s main interest and the center of the company’s attention. The AVALON-H90, with its existing VRDA system, is addressing a whole range of issues, including detection of the heart ventricle repolarization abnormalities that reflect several myocardial problems, including ischemia.
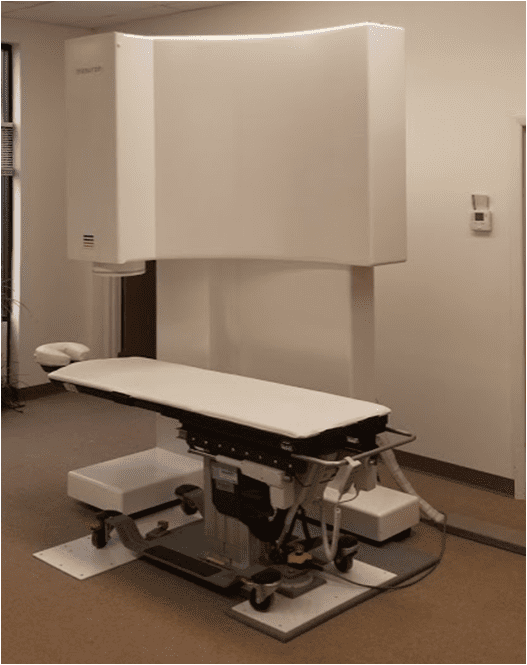
The AVALON-H90
Recently developed, the AVALON-H90 operates without the traditional use of liquid helium cryostats, using instead a proprietary chamber with an integrated cooling system. The device has a unique and revolutionary design, using single chip sensors based on SQUID technology. The sensor array is precisely fixed in a 3D orientation in the space above the human body. Such a configuration enables the AVALON-H90 to measure the heart’s electrical activity in all 3 space dimensions delivering measurement data that is impossible to obtain by any other known technology
- Measurements can be reliably made in close proximity, as close as 2.5 to 3 meters, to magnetic noise sources, such as hospital elevators, all kinds of medical apparatus, moving metal beds, working printers, and computers, without requiring a magnetically shielded enclosure. The AVALON-H90 has a small footprint, low power consumption, and no radiation or patient prep/medication.
- The AVALON-H90 is capable of providing a millisecond-by-millisecond high spatial resolution of the 3D electric activity maps over the human heart within ~ five minutes, with the patient resting. It requires no contact with the patient’s body. Due to its inherent synchronization design, the heart’s entire magnetic 3D map is created by using a single, completely contactless ~90-second measurement, this device will address a whole range of issues, including detection of the heart ventricle repolarization abnormalities that reflect several myocardial problems, including ischemia.
- Mesuron Inc. developed Ventricular Repolarization Dynamics Analysis algorithms that detect the multidimensional dynamics of the electrical activity caused by differences in the functions of Electrical Action Potential (“EAP”) of normal heart tissues and abnormal ones with hypoxia; it is also robust enough to detect abnormalities without hypoxia due to changes in muscle chemistry related to insufficient blood supply or other myocardial abnormalities.