MicroTransponder, Inc. today announced the United States Food and Drug Administration (FDA) Premarket Approval of the Vivistim Paired VNS™ System, which significantly improves the effectiveness of rehabilitation therapy for stroke survivors with moderate to severe upper extremity impairment six months after stroke. Every year, approximately 800,000 people in the United States have a stroke.1 Up to 60% of these survivors suffer from persistent impaired upper limb function six months following a stroke.2
“One of the most frustrating things for our patients who had a stroke is the inability to perform activities of daily living because of the weakness of the upper limb,” said Gerard Francisco, MD, professor, and chair of physical medicine and rehabilitation at McGovern Medical School at The University of Texas Health Science Center and Chief Medical Officer at TIRR Memorial Hermann, Houston, TX. “The current therapies we prescribe are quite limited. Many of these therapies have not been shown to induce the neuroplastic changes important for long-lasting recovery. We are excited to offer our patients the Vivistim System to magnify their potential to recover further.”
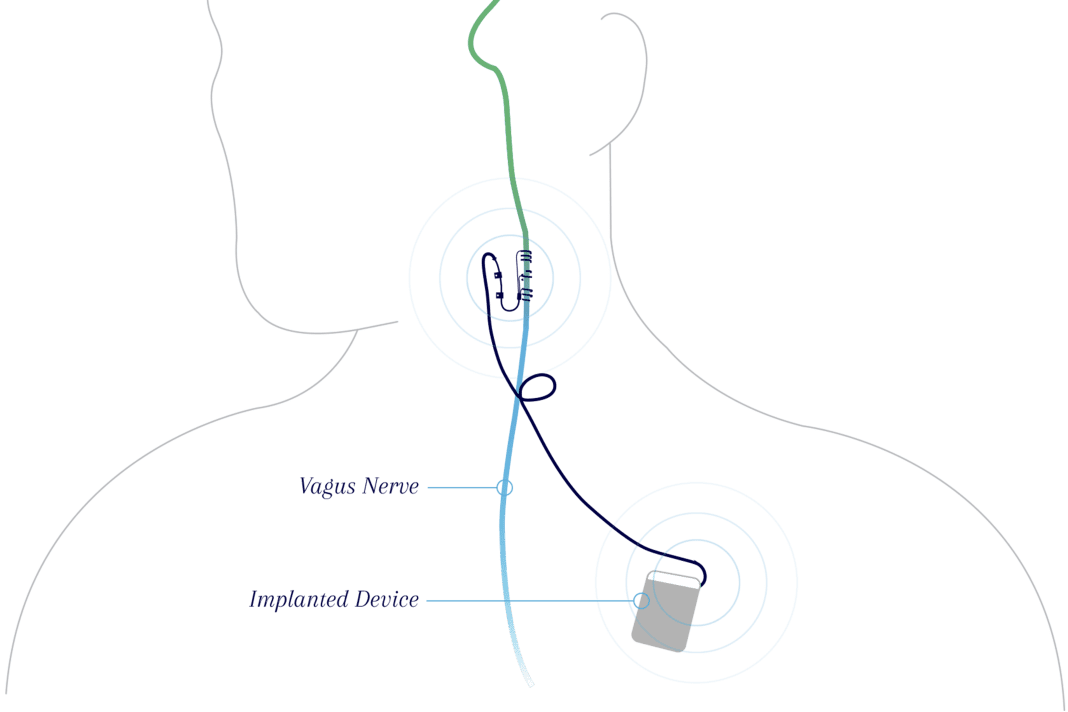
MicroTransponder’s platform pairs VNS with rehabilitation therapy to help improve stroke-related arm and hand deficits. The Vivistim neurostimulation device is placed just under the skin of the chest and neck in a short outpatient procedure. During in-clinic rehabilitation therapy, a therapist pairs gentle pulses of VNS with standard rehabilitation exercises. Pre-clinical data showed that VNS triggers the release of neuromodulators, making rehabilitation therapy more effective by strengthening the neural connections during the rehab exercises. The Vivistim System can also be activated by the user at home during rehabilitation exercises or activities of daily living they wish to improve.
Intense rehabilitation therapy is currently the best available treatment option for improving upper limb function after stroke. In the 108 subject VNS-REHAB pivotal study, published in The Lancet, Vivistim users showed statistically significant improvement in upper limb impairment and function as compared to intense rehabilitation therapy (<0.01).2 The study achieved its FDA-specified primary and secondary endpoints. Quality of life data showed 65% of Vivistim users reporting clinically significant improvement in their ability to perform activities of daily living.3
“More than a decade of preclinical research has shown that pairing vagus nerve stimulation with rehabilitation tasks strengthens neural connections and improves motor function,” noted Navzer Engineer, MD, Ph.D. and Chief Scientific Officer of MicroTransponder. “In our trial, Vivistim users showed a three-fold improvement in both arm and hand function compared to intense rehabilitation. Our therapy helped many chronic stroke survivors improve arm and hand function, allowing them to do activities that they were unable to do after their stroke. We look forward to researching ways to expand the Vivistim Paired VNS™ platform to help patients with other chronic neurologic conditions.”
The Vivistim System was granted a Breakthrough Device Designation by the FDA and will be available in targeted United States markets in late 2021, expanding nationally by the end of 2022. The VNS implantation procedure has been FDA-approved as safe and effective for over 20 years in other therapeutic areas.
References
References
- Virani SS et al. Heart disease and stroke statistics—2021 update: a report from the American Heart Association. Circulation. 2021;141(9):143:e254–e743
- Dawson et al. Vagus nerve stimulation paired with rehabilitation for upper limb motor function after ischaemic stroke (VNS-REHAB): a randomised, blinded, pivotal, device trial. Lancet. 2021; 397 1545–1553.
- MicroTransponder data on file.