By Dr. AG Stanley
It is sold exclusively by our company Stanley Medical Designs, Inc. We are an independent medical device company located in Miami Beach, Fla. The company’s mission is to make medical procedures safer, and more efficient and improve the patient care experience. Our company currently holds 5 medical device patents in these areas of interest.
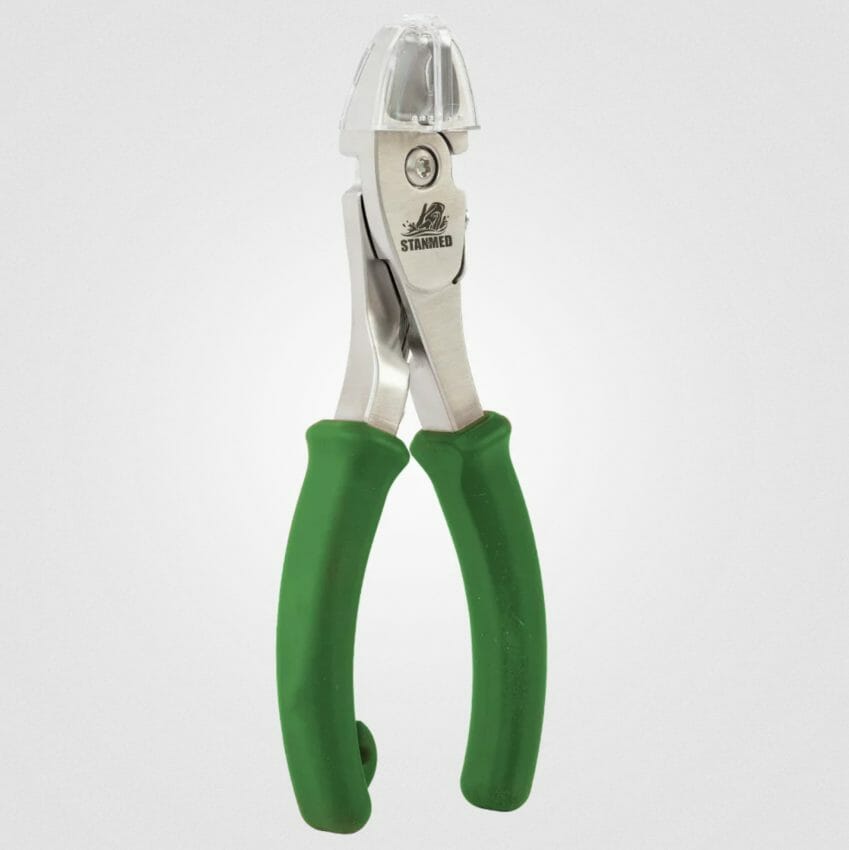 Our medical design team has over 35 years of experience performing surgical procedures in hospitals, emergency rooms, and urgent care systems. Our first offering is a new hybrid surgical wire cutter, called a Moby Cutter™. It is the first cutter of its kind, with a catchment device. This device can prevent cut pieces of material from becoming dangerous projectiles, which can puncture skin, endanger eyes or fall into the deep surgical field. It has been cleared for commercialization per FDA guidelines and sold as a prescription-only (Rx) medical device.
Our medical design team has over 35 years of experience performing surgical procedures in hospitals, emergency rooms, and urgent care systems. Our first offering is a new hybrid surgical wire cutter, called a Moby Cutter™. It is the first cutter of its kind, with a catchment device. This device can prevent cut pieces of material from becoming dangerous projectiles, which can puncture skin, endanger eyes or fall into the deep surgical field. It has been cleared for commercialization per FDA guidelines and sold as a prescription-only (Rx) medical device.
At 6 ½ inches tall, it muscles up to, the taller competition. The Moby Cutter™ features:
- 2 mm standard wire, cutting capacity
- Ergonomic handles for improved grip
- Dual action return spring to prevent hand fatigue
- Beveled blade edges allow closer cutting to the skin surface and avoid tissue damage
- Safety lock
- Surgical Steel composition
- Weight 0.25 Kg
- Optional single-use basket attachment, helps to prevent a Sentinel Event
- Totally autoclavable stylish device
- FDA Registered and listed as a class 1 medical device
Most distinctively it has a patented transparent basket attachment that prevents cut fragments from becoming skin and eye penetrating projectiles. The basket feature is called a Flick Catcher™. The cutter is available in a Surgical Green color.
The Moby Cutter™ has applications in:
- Orthopedic Surgery (Cutting K- wires and select Steinmann Pins)
- Hand Surgery (Cutting K- wires)
- Podiatry Surgery (Cutting K- wires)
- Foot and ankle Surgery (Cutting K- wires)
- Cardiothoracic Surgery (Cutting Sternal wires)
- Oral Facial Maxillary Surgery (Cutting jaw wires)
- Emergency Room (foreign body removal, cutting body jewelry and fishhooks)
- Urgent Care (foreign body removal, cutting body jewelry and fishhooks)
“The Moby Cutter™ has special recognition from the US Patent office, for usage in surgical fish hook removal procedures, which distinguishes it from the standard wire cutters currently being used.”
Lastly, the Moby Cutter™ has a sister cutter, Called the SoBe Cutter™, named in tribute to our company’s South Beach, Florida origin. Both cutters have similar features and function. The SoBe Cutters™ come in stunning Vice Blue or Vice Red colors.
Every surgeon must have one on their surgical tray!
Be one of the first to discover a new cutting experience!
Click here for more information. Phone: (305) 546-7784 Monday – Friday 9 am – 5 pm ET or Email: info@stanleymedicaldesigns.com
About AG Stanley, MD: Dr. Stanley is a Staff physician at Criticare Clinics & Urgent Care Miami, Fl. Staff Emergency Room Physician, Baptist Healthcare of South Florida. Medical Device Inventor (holder of 5 medical device patents) and Author. Dr. Stanley received his medical degree from Rutgers-New Jersey Medical School, Newark New Jersey. He completed his Residency in Internal Medicine at the University of Miami-Jackson Memorial Medical Center/ Miami VA Medical Center Miami, Florida.