Modulim, the global leader in optical imaging solutions for the non-invasive assessment of tissue and vascular health, announced today that Clarifi® has received CE Mark certification.
Modulim notes this allows the company to now expand its marketing and distribution throughout Europe and apply for registration in other international markets that recognize the CE Mark.
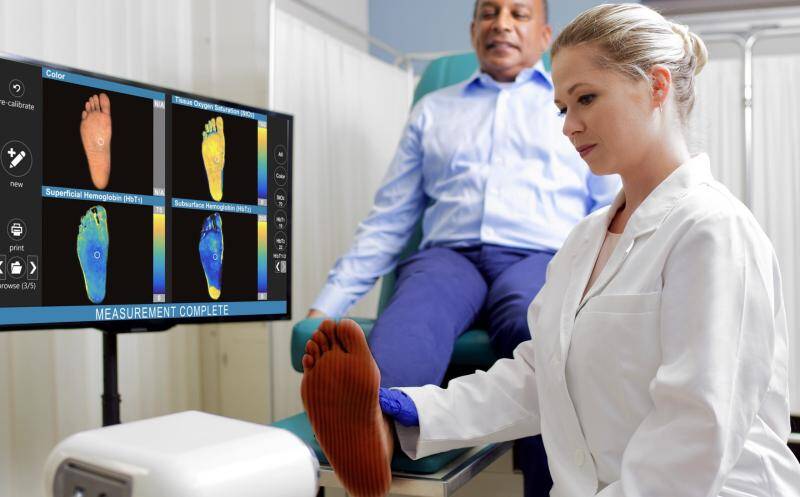
Powered by Spatial Frequency Domain Imaging (SFDI), a patented optical imaging technology, Clarifi is the newest advancement for assessing tissue and microvascular function by quantitatively mapping tissue oxygenation and perfusion at the point-of-care. Clarifi’s fully-integrated solution is rapidly gaining acceptance by clinicians across the continuum of care for assessing potential circulatory compromise to manage and treat diabetic, venous and pressure ulcers, acute and chronic wounds, burns, and amputations.
“Obtaining CE marking for the Clarifi Imaging System is a key milestone for Modulim,” said David Cuccia, Ph.D., Modulim’s President and CTO. “This achievement allows us to expand our addressable market and deliver a complete microvascular assessment solution that helps clinicians prevent lower limb complications in patients with diabetes as well as other life-altering circulatory issues. We are now able to bring our innovative technology to our European clinical partners, and grow our ever expanding image library by reaching the 60 million diabetes patients in the EU.”
Clarifi can help clinicians identify potential compromised circulation and pre-ulcerative conditions, before they are visible, so they can implement timely and targeted intervention to prevent costly and deadly complications. Powered by SFDI, Clarifi provides new information that enables healthcare providers in all environments to transition from reactive care to proactive care.
The Clarifi Imaging System demonstrates Modulim’s continuing mission to deliver transformative optical solutions that help people live healthier, longer lives.